Another great update on the vaccine landscape by Derek Lowe today in his In The Pipeline blog in Science Translational Medicine. He's done several updates on individual vaccines over the past month or two, but this is his first comprehensive update in almost two months. He does a very nice job going through all the various vaccine categories and candidates in each category, with significant focus on the 9 in phase III and others already in early clinical trials. The NY Times also has a nice vaccine tracker, which is visually better organized than Lowe's effort and it contains a nice overview of the vaccine development process, but it's not nearly as detailed around the science of the vaccines. Lowe also has embedded links to all of his more in-depth blogs on individual vaccines and animal/human results to date.

blogs.sciencemag.org
A look at all the vaccines that have reached trials in humans.
www.nytimes.com
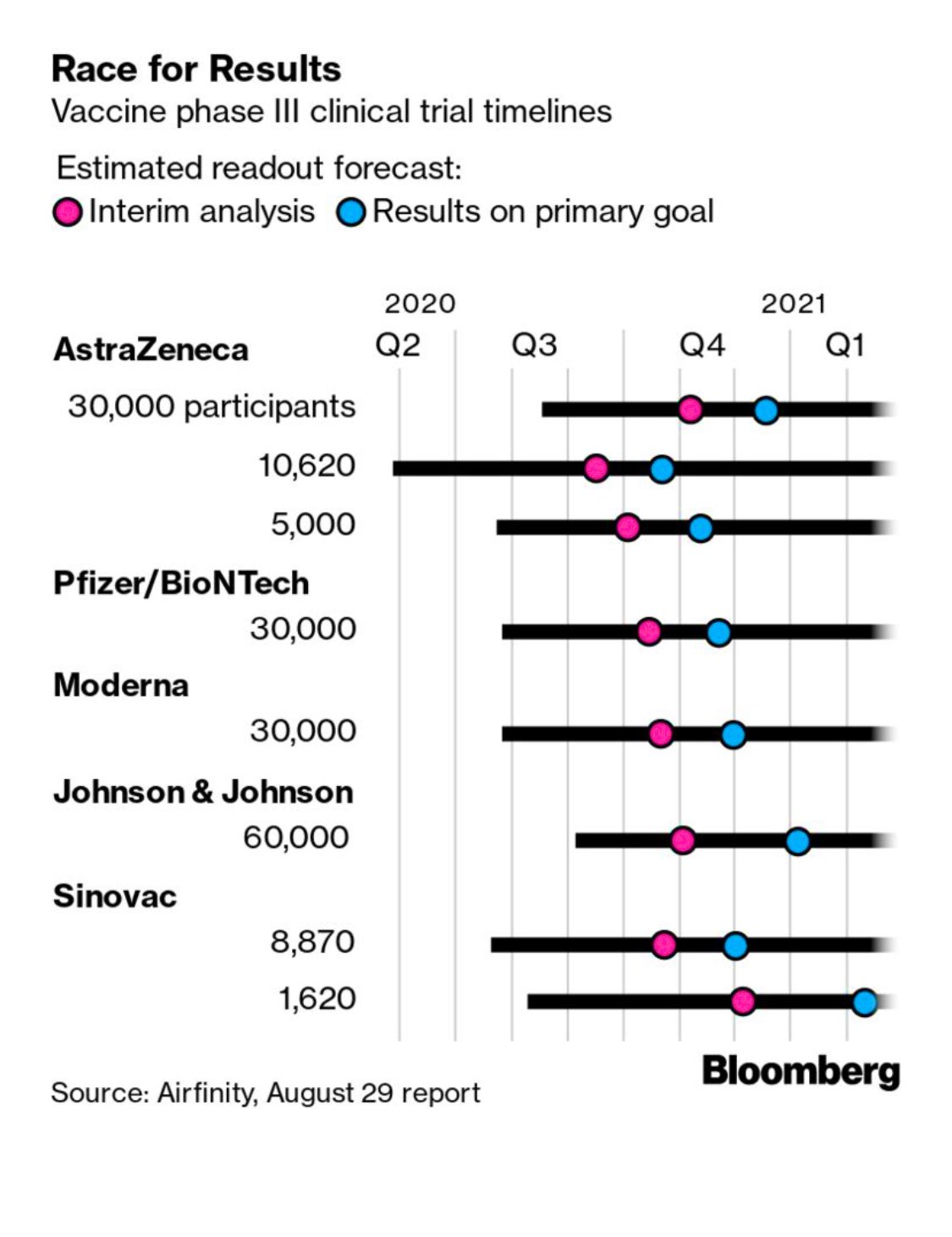
I'm not going to try to repeat the discussions in either link, other than to say that we're really getting down to brass tacks soon, with the pivotal phase III trial results likely only about 2 months away and while I'm optimistic we'll have an approved vaccine by the end of the year (been saying that since April), I think people talking about approvals by the end of October are kidding themselves, given how much data are being generated and how much there will be to review prior to approval. I have listed the 10 vaccines in phase III, below for easy reference and all of them have had reasonably good safety profiles and implied efficacy (prompting good immune responses), so far, meaning no major red flags, but obviously in smaller phase II trials w/o any data on conferring immunity yet.
Keep in mind that all of the vaccines in phase II or III right now will require an initial shot and then a booster shot (typically a month later) to achieve optimal immunity, with the exception of the J&J (Janssen) vaccine which is looking at a single shot. Some of the vaccines that are a few months behind the frontrunners, like Novavax and the Merck/Themis vaccine are looking at single shots and possibly greater immunity levels. Also, remember that the FDA is saying a vaccine is "effective" if it can "prevent disease or decrease its severity in at least 50% of people who are vaccinated," so it's possible efficacy will look similar to the flu vaccine (although most experts expect much greater than 50% protection).
- Moderna's mRNA vaccine; no mRNA vaccine has ever been approved before; requires -20C cold chain (freezers)
- Pfizer/BioNTech's mRNA vaccine - requires -70C cold chain for stability of the formulation (dry ice storage typically - probably not a huge issue in first world countries, but a major issue elsewhere)
- Astra Zeneca/Oxford's chimp adenovirus viral vector vaccine (only 1 vvv has been approved - Merck's Ebola vaccine)
- CanSino's human adenovirus viral vector vaccine; human adenoviruses might have pre-existing immunity issues
- J&J's human adenovirus viral vector vaccine (using an obscure human virus, hoping to avoid the pre-existing immunity issues); this one starts phase III any day now, so I included it.
- Russia's Gamaleya Institute's human adenovirus viral vector vaccine (already "approved" in Russia - a PR stunt)
- There are three Chinese vaccines using "old school" deactivated actual coronavirus (most vaccines in use today use this approach, but it's much harder to scale and often less potent, but it works usually); two of these also have "emergency use approvals" without phase III data, which is just dumb.
- The last one is an oddball. It's a trial of the existing Bacillus Calmette-Guerin vaccine for tuberculosis in Australia.
In addition, here are a few more interesting links on some key issues for these vaccines. The first one is another Lowe blog entry, this time on what exactly "cold chain" means for vaccine stability, supply chains, and availability, especially in the 3rd world. The 2nd one is also a Lowe blog, on what can sound like a mundane topic, but it really isn't, as it's on things like ensuring there are enough glass vials or silica-coated vials for billions of doses and ensuring that the vials used are gas tight (a key for stability), which is no trivial task. I've done a lot of work in both of these areas over the years for new pharmaceuticals and they're not as "sexy" as the actual product, but if mishandled, they can derail an entire product launch.

blogs.sciencemag.org

blogs.sciencemag.org
Lastly, we should talk at least a bit about rumors of premature approvals or emergency use without appropriately vetted safety and efficacy data. Clearly, there is immense pressure to get some great vaccines out there as quickly as possible without cutting important corners (as there should be with lives at stake every day). My sincere hope is that this should be a moot point if the scientists at Warp Speed, NIH, FDA, etc. are making the decisions and I liked what I've heard from both Fauci and Slaoui (Warp Speed scientific lead) lately with regard to them not cutting corners to meet any artificial deadline (election day, for example) - if something is safe and effective by then, great, but if it's not, we wait for that day. The only thing I could see happening "early" is a potential "emergency use authorization" for early use in highly vulnerable people (health care workers in locations with outbreaks). I simply can't imagine this being "approved" for the public before full data on safety and efficacy are available from phase III trials. It would simply be monumentally stupid - and there's no way many would participate. We're not Russia or China.

www.sciencemag.org
Dr. Moncef Slaoui, chief adviser to the administration's effort to develop a COVID-19 vaccine, said having a vaccine by next month was "not impossible." But a longer timetable appears more likely.

www.npr.org
Edit: I liked reading that the Pfizer and Merck CEO's both came out and said their companies simply won't consider filing for approval for a vaccine without having completed a successful phase III trial. That is excellent news for vaccine safety and efficacy.
https://www.livemint.com/news/world...gulatory-nod-for-vaccines-11599147880530.html