I've seen Caddyshack at least 50X and I don't drink martinis, so maybe I'll just continue trying to provide relevant info. No idea what you think is wrong with a post correcting someone who was completely wrong, but now I know why lions eat their young.Really like your weather posts but now you are getting carried away with yourself - Have a nice martini and watch caddy shack.
Colleges
- AAC
- ACC
- Big 12
- Big East
- Big Ten
- Pac-12
- SEC
- Atlantic 10
- Conference USA
- Independents
- Junior College
- Mountain West
- Sun Belt
- MAC
- More
- Navy
- UAB
- Tulsa
- UTSA
- Charlotte
- Florida Atlantic
- Temple
- Rice
- East Carolina
- USF
- SMU
- North Texas
- Tulane
- Memphis
- Miami
- Louisville
- Virginia
- Syracuse
- Wake Forest
- Duke
- Boston College
- Virginia Tech
- Georgia Tech
- Pittsburgh
- North Carolina
- North Carolina State
- Clemson
- Florida State
- Cincinnati
- BYU
- Houston
- Iowa State
- Kansas State
- Kansas
- Texas
- Oklahoma State
- TCU
- Texas Tech
- Baylor
- Oklahoma
- UCF
- West Virginia
- Wisconsin
- Penn State
- Ohio State
- Purdue
- Minnesota
- Iowa
- Nebraska
- Illinois
- Indiana
- Rutgers
- Michigan State
- Maryland
- Michigan
- Northwestern
- Arizona State
- Oregon State
- UCLA
- Colorado
- Stanford
- Oregon
- Arizona
- California
- Washington
- USC
- Utah
- Washington State
- Texas A&M
- Auburn
- Mississippi State
- Kentucky
- South Carolina
- Arkansas
- Florida
- Missouri
- Ole Miss
- Alabama
- LSU
- Georgia
- Vanderbilt
- Tennessee
- Louisiana Tech
- New Mexico State
- Middle Tennessee
- Western Kentucky
- UTEP
- Florida International University
High School
- West
- Midwest
- Northeast
- Southeast
- Other
- Alaska
- Arizona
- California
- Colorado
- Nevada
- New Mexico
- Northern California
- Oregon
- Southern California Preps
- Washington
- Edgy Tim
- Indiana
- Kansas
- Nebraska
- Iowa
- Michigan
- Minnesota
- Missouri
- Oklahoma Varsity
- Texas Basketball
- Texas
- Wisconsin
- Delaware
- Maryland
- New Jersey Basketball
- New Jersey
- New York City Basketball
- Ohio
- Pennsylvania
- Greater Cincinnati
- Virginia
- West Virginia Preps
ADVERTISEMENT
Install the app
How to install the app on iOS
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
OT: COVID Science - Pfizer/Moderna vaccines >90% effective; Regeneron antibody cocktail looks very promising in phase II/III trial and more
- Thread starter RU848789
- Start date
- Status
- Not open for further replies.
You completely whiffed on statistical significance and now you're going to double down telling me I'm wrong about saying the interim analysis at 50% enrollment by the data safety monitoring board, whose primary focus is ensuring patient safety, was about clinical efficacy? Good luck with that. There are usually three possible outcomes from a DSMB review: i) evaluation of safety in the treatment arm vs. the placebo arm, with termination of the trial if excess adverse events in the treatment arm; ii) termination of the trial if the clinical outcome, to that point, is overwhelmingly positive, and iii) stopping the trial early due to "futility" i.e., it's obvious there is unlikely to be significant efficacy.Once again you are wrong. The DSMB first look at the severe /critical trial at 149 patients was a safety look. Pass with flying colors. The next look at 195 patients was an efficacy look. Passed again , no stoppage or change of trial. Why would the DSMB ask for another look at 293 patients when the trial is designed for 390 patients. The only conclusion is that although it is critically significant it would not be statistically significant until 293. Extremely unusual for DSMB to suggest a look at lower numbers instead of adding more patients to increase chances of statistical significance . What is your take ?
So, the decision to proceed means there was no bad safety signal, which is good, the clinical data are not overwhelmingly positive (which is a likely outcome halfway through most trials), and there is likely at least some signal of efficacy, but it's not statistically significant, so the trial needs to proceed to gain enough data to determine that significance. This is a typical outcome and does not mean the trial, at the endpoint, will be shown to be both safe and effective or not - we simply don't know yet. I continue to hope the trial shows efficacy, but to say we have relevant data yet is simply wrong.
https://en.wikipedia.org/wiki/Data_monitoring_committee
My bet since March was that Regeneron's antibody cocktail (mix of two monoclonal antibodies that target two different epitopes on the virus's spike protein) was going to be our best hope for seriously improving patient response to being infected and that seems like what we're seeing with today's additional results from the ongoing phase II/III trial in mild to moderately ill COVID patients (prior to hospitalization, when an antiviral is likely to have the biggest benefit).
Certainly can't say it's a "cure" yet, but it's looking much better than any other drug out there, so far and could be a bridge to vaccines, especially as it's also being looked at as a prophylactic to prevent infection in high risk workers/possible patients. Regeneron has applied with the FDA for an Emergency Use Authorization soon, which I think makes sense. Yeah, I know - no COVID threads, but people ought to know about this. At least leave it up for a day or so. This is really important news given that Eli Lilly announced that they were stopping their trial in hospitalized COVID patients getting their single antibody treatment along with remdesivir, due to lack of efficacy (they also have a cocktail - awaiting results on that).
https://investor.regeneron.com/news...9-outpatient-trial-prospectively-demonstrates
𝗥𝗲𝗴𝗲𝗻𝗲𝗿𝗼𝗻 𝗣𝗵𝗮𝗿𝗺𝗮𝗰𝗲𝘂𝘁𝗶𝗰𝗮𝗹𝘀, 𝗜𝗻𝗰. (𝗡𝗔𝗦𝗗𝗔𝗤: 𝗥𝗘𝗚𝗡) 𝘁𝗼𝗱𝗮𝘆 𝗮𝗻𝗻𝗼𝘂𝗻𝗰𝗲𝗱 𝗽𝗼𝘀𝗶𝘁𝗶𝘃𝗲, 𝗽𝗿𝗼𝘀𝗽𝗲𝗰𝘁𝗶𝘃𝗲 𝗿𝗲𝘀𝘂𝗹𝘁𝘀 𝗳𝗿𝗼𝗺 𝗮𝗻 𝗼𝗻𝗴𝗼𝗶𝗻𝗴 𝗣𝗵𝗮𝘀𝗲 𝟮/𝟯 𝘀𝗲𝗮𝗺𝗹𝗲𝘀𝘀 𝘁𝗿𝗶𝗮𝗹 𝗶𝗻 𝘁𝗵𝗲 𝗖𝗢𝗩𝗜𝗗-𝟭𝟵 𝗼𝘂𝘁𝗽𝗮𝘁𝗶𝗲𝗻𝘁 𝘀𝗲𝘁𝘁𝗶𝗻𝗴 𝘀𝗵𝗼𝘄𝗶𝗻𝗴 𝗶𝘁𝘀 𝗶𝗻𝘃𝗲𝘀𝘁𝗶𝗴𝗮𝘁𝗶𝗼𝗻𝗮𝗹 𝗮𝗻𝘁𝗶𝗯𝗼𝗱𝘆 𝗰𝗼𝗰𝗸𝘁𝗮𝗶𝗹, 𝗥𝗘𝗚𝗡-𝗖𝗢𝗩𝟮, 𝗺𝗲𝘁 𝘁𝗵𝗲 𝗽𝗿𝗶𝗺𝗮𝗿𝘆 𝗮𝗻𝗱 𝗸𝗲𝘆 𝘀𝗲𝗰𝗼𝗻𝗱𝗮𝗿𝘆 𝗲𝗻𝗱𝗽𝗼𝗶𝗻𝘁𝘀. 𝗥𝗘𝗚𝗡-𝗖𝗢𝗩𝟮 𝘀𝗶𝗴𝗻𝗶𝗳𝗶𝗰𝗮𝗻𝘁𝗹𝘆 𝗿𝗲𝗱𝘂𝗰𝗲𝗱 𝘃𝗶𝗿𝗮𝗹 𝗹𝗼𝗮𝗱 𝗮𝗻𝗱 𝗽𝗮𝘁𝗶𝗲𝗻𝘁 𝗺𝗲𝗱𝗶𝗰𝗮𝗹 𝘃𝗶𝘀𝗶𝘁𝘀 (𝗵𝗼𝘀𝗽𝗶𝘁𝗮𝗹𝗶𝘇𝗮𝘁𝗶𝗼𝗻𝘀, 𝗲𝗺𝗲𝗿𝗴𝗲𝗻𝗰𝘆 𝗿𝗼𝗼𝗺, 𝘂𝗿𝗴𝗲𝗻𝘁 𝗰𝗮𝗿𝗲 𝘃𝗶𝘀𝗶𝘁𝘀 𝗮𝗻𝗱/𝗼𝗿 𝗽𝗵𝘆𝘀𝗶𝗰𝗶𝗮𝗻 𝗼𝗳𝗳𝗶𝗰𝗲/𝘁𝗲𝗹𝗲𝗺𝗲𝗱𝗶𝗰𝗶𝗻𝗲 𝘃𝗶𝘀𝗶𝘁𝘀).
"𝗧𝗵𝗲 𝗳𝗶𝗿𝘀𝘁 𝗷𝗼𝗯 𝗼𝗳 𝗮𝗻 𝗮𝗻𝘁𝗶𝘃𝗶𝗿𝗮𝗹 𝘁𝗵𝗲𝗿𝗮𝗽𝗲𝘂𝘁𝗶𝗰 𝗱𝗿𝘂𝗴 𝗶𝘀 𝘁𝗼 𝗹𝗼𝘄𝗲𝗿 𝘁𝗵𝗲 𝘃𝗶𝗿𝗮𝗹 𝗹𝗼𝗮𝗱, 𝗮𝗻𝗱 𝗼𝘂𝗿 𝗶𝗻𝗶𝘁𝗶𝗮𝗹 𝗱𝗮𝘁𝗮 𝗶𝗻 𝟮𝟳𝟱 𝗽𝗮𝘁𝗶𝗲𝗻𝘁𝘀 𝘀𝘁𝗿𝗼𝗻𝗴𝗹𝘆 𝘀𝘂𝗴𝗴𝗲𝘀𝘁𝗲𝗱 𝘁𝗵𝗮𝘁 𝘁𝗵𝗲 𝗥𝗘𝗚𝗡-𝗖𝗢𝗩𝟮 𝗮𝗻𝘁𝗶𝗯𝗼𝗱𝘆 𝗰𝗼𝗰𝗸𝘁𝗮𝗶𝗹 𝗰𝗼𝘂𝗹𝗱 𝗹𝗼𝘄𝗲𝗿 𝘃𝗶𝗿𝗮𝗹 𝗹𝗼𝗮𝗱 𝗮𝗻𝗱 𝘁𝗵𝗲𝗿𝗲𝗯𝘆 𝗽𝗼𝘁𝗲𝗻𝘁𝗶𝗮𝗹𝗹𝘆 𝗶𝗺𝗽𝗿𝗼𝘃𝗲 𝗰𝗹𝗶𝗻𝗶𝗰𝗮𝗹 𝗼𝘂𝘁𝗰𝗼𝗺𝗲𝘀. 𝗧𝗼𝗱𝗮𝘆'𝘀 𝗮𝗻𝗮𝗹𝘆𝘀𝗶𝘀, 𝗶𝗻𝘃𝗼𝗹𝘃𝗶𝗻𝗴 𝗺𝗼𝗿𝗲 𝘁𝗵𝗮𝗻 𝟱𝟬𝟬 𝗮𝗱𝗱𝗶𝘁𝗶𝗼𝗻𝗮𝗹 𝗽𝗮𝘁𝗶𝗲𝗻𝘁𝘀, 𝗽𝗿𝗼𝘀𝗽𝗲𝗰𝘁𝗶𝘃𝗲𝗹𝘆 𝗰𝗼𝗻𝗳𝗶𝗿𝗺𝘀 𝘁𝗵𝗮𝘁 𝗥𝗘𝗚𝗡-𝗖𝗢𝗩𝟮 𝗰𝗮𝗻 𝗶𝗻𝗱𝗲𝗲𝗱 𝘀𝗶𝗴𝗻𝗶𝗳𝗶𝗰𝗮𝗻𝘁𝗹𝘆 𝗿𝗲𝗱𝘂𝗰𝗲 𝘃𝗶𝗿𝗮𝗹 𝗹𝗼𝗮𝗱 𝗮𝗻𝗱 𝗳𝘂𝗿𝘁𝗵𝗲𝗿 𝘀𝗵𝗼𝘄𝘀 𝘁𝗵𝗮𝘁 𝘁𝗵𝗲𝘀𝗲 𝘃𝗶𝗿𝗮𝗹 𝗿𝗲𝗱𝘂𝗰𝘁𝗶𝗼𝗻𝘀 𝗮𝗿𝗲 𝗮𝘀𝘀𝗼𝗰𝗶𝗮𝘁𝗲𝗱 𝘄𝗶𝘁𝗵 𝗮 𝘀𝗶𝗴𝗻𝗶𝗳𝗶𝗰𝗮𝗻𝘁 𝗱𝗲𝗰𝗿𝗲𝗮𝘀𝗲 𝗶𝗻 𝘁𝗵𝗲 𝗻𝗲𝗲𝗱 𝗳𝗼𝗿 𝗳𝘂𝗿𝘁𝗵𝗲𝗿 𝗺𝗲𝗱𝗶𝗰𝗮𝗹 𝗮𝘁𝘁𝗲𝗻𝘁𝗶𝗼𝗻," 𝘀𝗮𝗶𝗱 𝗚𝗲𝗼𝗿𝗴𝗲 𝗗. 𝗬𝗮𝗻𝗰𝗼𝗽𝗼𝘂𝗹𝗼𝘀, 𝗠.𝗗., 𝗣𝗵.𝗗., 𝗣𝗿𝗲𝘀𝗶𝗱𝗲𝗻𝘁 𝗮𝗻𝗱 𝗖𝗵𝗶𝗲𝗳 𝗦𝗰𝗶𝗲𝗻𝘁𝗶𝗳𝗶𝗰 𝗢𝗳𝗳𝗶𝗰𝗲𝗿 𝗼𝗳 𝗥𝗲𝗴𝗲𝗻𝗲𝗿𝗼𝗻. "𝗪𝗲 𝗰𝗼𝗻𝘁𝗶𝗻𝘂𝗲 𝘁𝗼 𝘀𝗲𝗲 𝘁𝗵𝗲 𝘀𝘁𝗿𝗼𝗻𝗴𝗲𝘀𝘁 𝗲𝗳𝗳𝗲𝗰𝘁𝘀 𝗶𝗻 𝗽𝗮𝘁𝗶𝗲𝗻𝘁𝘀 𝘄𝗵𝗼 𝗮𝗿𝗲 𝗺𝗼𝘀𝘁 𝗮𝘁 𝗿𝗶𝘀𝗸 𝗳𝗼𝗿 𝗽𝗼𝗼𝗿 𝗼𝘂𝘁𝗰𝗼𝗺𝗲𝘀 𝗱𝘂𝗲 𝘁𝗼 𝗵𝗶𝗴𝗵 𝘃𝗶𝗿𝗮𝗹 𝗹𝗼𝗮𝗱, 𝗶𝗻𝗲𝗳𝗳𝗲𝗰𝘁𝗶𝘃𝗲 𝗮𝗻𝘁𝗶𝗯𝗼𝗱𝘆 𝗶𝗺𝗺𝘂𝗻𝗲 𝗿𝗲𝘀𝗽𝗼𝗻𝘀𝗲 𝗮𝘁 𝗯𝗮𝘀𝗲𝗹𝗶𝗻𝗲, 𝗼𝗿 𝗽𝗿𝗲-𝗲𝘅𝗶𝘀𝘁𝗶𝗻𝗴 𝗿𝗶𝘀𝗸 𝗳𝗮𝗰𝘁𝗼𝗿𝘀. 𝗥𝗲𝗴𝗲𝗻𝗲𝗿𝗼𝗻 𝗵𝗮𝘀 𝘀𝗵𝗮𝗿𝗲𝗱 𝘁𝗵𝗲𝘀𝗲 𝗿𝗲𝘀𝘂𝗹𝘁𝘀 𝘄𝗶𝘁𝗵 𝘁𝗵𝗲 𝗨.𝗦. 𝗙𝗼𝗼𝗱 𝗮𝗻𝗱 𝗗𝗿𝘂𝗴 𝗔𝗱𝗺𝗶𝗻𝗶𝘀𝘁𝗿𝗮𝘁𝗶𝗼𝗻 𝗮𝘀 𝗽𝗮𝗿𝘁 𝗼𝗳 𝗶𝘁𝘀 𝗿𝗲𝘃𝗶𝗲𝘄 𝗼𝗳 𝗼𝘂𝗿 𝗘𝗺𝗲𝗿𝗴𝗲𝗻𝗰𝘆 𝗨𝘀𝗲 𝗔𝘂𝘁𝗵𝗼𝗿𝗶𝘇𝗮𝘁𝗶𝗼𝗻 𝘀𝘂𝗯𝗺𝗶𝘀𝘀𝗶𝗼𝗻, 𝗮𝗻𝗱 𝘄𝗲 𝗰𝗼𝗻𝘁𝗶𝗻𝘂𝗲 𝘁𝗼 𝗳𝗼𝗰𝘂𝘀 𝗼𝗻 𝗰𝗼𝗺𝗽𝗹𝗲𝘁𝗶𝗻𝗴 𝗼𝘂𝗿 𝗼𝗻𝗴𝗼𝗶𝗻𝗴 𝘁𝗿𝗶𝗮𝗹𝘀 𝗲𝘃𝗮𝗹𝘂𝗮𝘁𝗶𝗻𝗴 𝗥𝗘𝗚𝗡-𝗖𝗢𝗩𝟮 𝗳𝗼𝗿 𝘁𝗵𝗲 𝘁𝗿𝗲𝗮𝘁𝗺𝗲𝗻𝘁 𝗮𝗻𝗱 𝗽𝗿𝗲𝘃𝗲𝗻𝘁𝗶𝗼𝗻 𝗼𝗳 𝗖𝗢𝗩𝗜𝗗-𝟭𝟵."
Here's a follow-up article on the antibody treatment approach by Regeneron and Eli Lilly from my favorite science blogger, Derek Lowe in ScienceMag (first link; 2nd link is to an Endpoints discussion of the Lilly data), as he addressed the same study I did last night, with similar comments for the most part. He noted that the Regeneron data on significant reductions in viral loads and need for medical intervention in mildly to moderately ill COVID patients was very good news, but also noted that we're not going to have nearly enough doses for everyone who might want this treatment, this year and even next year will likely be tight (if we only treat US patients - I posted on that this past afternoon).
https://blogs.sciencemag.org/.../the-latest-antibody-data...
https://endpts.com/a-p-value-of-0-38-nejm-results-raise.../
That's why we need Eli Lilly's antibody cocktail to also work, as well as antibody treatments from others, especially since we're not going to have nearly enough people vaccinated to prevent large numbers of infections well into next year and will need more effective treatments. So, he also commented on the Lilly trial with their single antibody, which was somewhat disappointing, as per results shared a few weeks ago, except now they've published their paper on it in the New England Journal of Medicine.
There was clinical efficacy seen in viral load reduction in mildly ill COVID patients at the mid-dose (2800 mg), but not at the target dose of 700 mg - yet, Lilly wants to get an EUA (emergency use authorization) on the 700 mg dose, which many are questioning. In addition this single antibody showed no efficacy in sicker, hospitalized patients, so far, when combined with remdesivir (link below). It's possible that a cocktail of two or more antibodies, like Regeneron's product, or Lilly's other product which is also in clinical trials and looks promising so far in mild/moderately ill COVID patients (2nd link below), is needed for significant efficacy. More to come, obviously.
https://www.npr.org/sections/corona...0mNb47yQ61SdJTJQ8pVG4NAWc1xDU84DgpOY9ZLrYlLfM
https://www.statnews.com/2020/10/07...jD2fyjpNZz7IThSwLSGs4QiCpIuys2HRzGUMFMj3oL0R4
Because there isn't any clear demonstration of efficacy. I could generate a positive safety profile in lots of patients with a placebo.Why do you think Leronlimab has not been approved for at least EUA yet when there has not been a negative impact of the drug in any trial, or EINd patients or over 1000 AIDS patients, so totally safe with no side effects. Enlighten us with your reasons !
Don't know if this was posted already, but this site summarized the continuing research into potential treatments and vaccines.

 covid-19tracker.milkeninstitute.org
covid-19tracker.milkeninstitute.org

Milken Institute’s COVID-19 Treatment and Vaccine Tracker tracks the development of treatments and vaccines for COVID-19 at covid-19tracker.milkeninstitute.org #COVID19 #coronavirus #COVID19treatment #COVID19vaccine @MilkenInstitute @FirstPersonSF
The Milken Institute’s COVID-19 Treatment and Vaccine Tracker tracks the development of treatments and vaccines for COVID-19 (coronavirus). Explore the latest updates on the urgent race to develop a COVID-19 vaccine.
 covid-19tracker.milkeninstitute.org
covid-19tracker.milkeninstitute.org
Well more news out. DSMB recommendations for Leronlimab vs. Regeneron show Leronlimab better. Regeneron was advised not to use Regeneron cocktail told to stop using any further high flow oxygen and mechanical ventilation patients into their clinical trial. Something not said and not an exclusion criteria in the Leronlimab trial. That leaves only Leronlimab for severe critical population in a clinical trial. Numbers why don’t you check again about the DSMB. The first look at the severe / critical trial was at 149 patients and that was a safety look. The second look at 195, at 50 % of the 390 designed enrollment , was an efficacy look , with recommendations to take another look at 293 patients . Are you really trying to tell me that wasn’t an efficacy recommendation ? I will ask you the same question I asked Cheese, why do you think they asked for another look at 293 before the full 390 designed to complete the trial? How often does the DSMB recommend another look before the complete trial is over instead of telling the company to add more patients or change their endpoint to succeed in their trial? Like they have done for the other Covid companies trying therapeutics, where they advised to stop the trial for futility, being unsafe, or not going to meet endpoint u less they change the trial !You completely whiffed on statistical significance and now you're going to double down telling me I'm wrong about saying the interim analysis at 50% enrollment by the data safety monitoring board, whose primary focus is ensuring patient safety, was about clinical efficacy? Good luck with that. There are usually three possible outcomes from a DSMB review: i) evaluation of safety in the treatment arm vs. the placebo arm, with termination of the trial if excess adverse events in the treatment arm; ii) termination of the trial if the clinical outcome, to that point, is overwhelmingly positive, and iii) stopping the trial early due to "futility" i.e., it's obvious there is unlikely to be significant efficacy.
So, the decision to proceed means there was no bad safety signal, which is good, the clinical data are not overwhelmingly positive (which is a likely outcome halfway through most trials), and there is likely at least some signal of efficacy, but it's not statistically significant, so the trial needs to proceed to gain enough data to determine that significance. This is a typical outcome and does not mean the trial, at the endpoint, will be shown to be both safe and effective or not - we simply don't know yet. I continue to hope the trial shows efficacy, but to say we have relevant data yet is simply wrong.
https://en.wikipedia.org/wiki/Data_monitoring_committee
Unfortunately, the Regeneron antibody cocktail trial in hospitalized patients was put on hold for those patients on ventilators or high flow oxygen, by the data safety monitoring board, due to a potential "safety signal." The trial has been modified to exclude those patients while the data on those patients is further evaluated (no word as to what the signal was), but will go on for the remainder of the hospitalized patients. This does not impact the ongoing trials in mild/moderately ill COVID patients (not in hospitalized) or those not infected, evaluating the possible prophylactic effect.
This is why trials have DSMBs - to ensure patient safety. Certainly a setback, but it's not clear if it's a permanent setback or not, plus, almost any antiviral is likely to work best before patients become seriously ill, so this is not a complete surprise.
https://investor.regeneron.com/news...ependent-data-monitoring-committee-recommends
https://endpts.com/in-a-second-big-...on-halts-enrollment-for-more-severe-patients/
This is why trials have DSMBs - to ensure patient safety. Certainly a setback, but it's not clear if it's a permanent setback or not, plus, almost any antiviral is likely to work best before patients become seriously ill, so this is not a complete surprise.
https://investor.regeneron.com/news...ependent-data-monitoring-committee-recommends
https://endpts.com/in-a-second-big-...on-halts-enrollment-for-more-severe-patients/
It could be because efficacy data is trending in the right direction. It could also be because it's trending in the opposite direction, because more interim looks are better given situation of the world / changing treatment paradigms, because the company wanted to add another look despite however it's messaged, because DSMB get paid to do these so perhaps they recommended another one and company said yes, etc. Could be million things.Well more news out. DSMB recommendations for Leronlimab vs. Regeneron show Leronlimab better. Regeneron was advised not to use Regeneron cocktail told to stop using any further high flow oxygen and mechanical ventilation patients into their clinical trial. Something not said and not an exclusion criteria in the Leronlimab trial. That leaves only Leronlimab for severe critical population in a clinical trial. Numbers why don’t you check again about the DSMB. The first look at the severe / critical trial was at 149 patients and that was a safety look. The second look at 195, at 50 % of the 390 designed enrollment , was an efficacy look , with recommendations to take another look at 293 patients . Are you really trying to tell me that wasn’t an efficacy recommendation ? I will ask you the same question I asked Cheese, why do you think they asked for another look at 293 before the full 390 designed to complete the trial? How often does the DSMB recommend another look before the complete trial is over instead of telling the company to add more patients or change their endpoint to succeed in their trial? Like they have done for the other Covid companies trying therapeutics, where they advised to stop the trial for futility, being unsafe, or not going to meet endpoint u less they change the trial !
Put it this way... stock market isn't viewing it as a sign of good news.
So we went from “ looking promising” to put on hold... all due respect to the science board gurus. How about we all wait until there is a actual valid break through. This is why people become hyper critical and skeptical. TRUTH... there will be no vaccine at 65-70% ... maybe some alternative interventional meds but a true workable vaccine is years away. This will be an every year AIDS vaccine debacle. How many years? Feel bad for those in the middle age groups.For those that will try to convince us the vaccine or vaccines are here by Spring... better not hold your breath.
There is a DMSB for every trial, so nobody has compared the Regeneron antibody cocktail vs. Leronlimab. Leronlimab may end up being safer for seriously ill, hospitalized patients, but we don't know that yet (the Regeneron trial was put on "hold" in patients on ventilators or with high flow O2, not stopped, so we don't know if it will continue). And trials at an interim point are occasionally asked by DMSB's to look at a further interim point downstream before the endpoint - neither you nor I "know" why they did that in Leronlimab's case (only the DSMB is unblinded to the data).Well more news out. DSMB recommendations for Leronlimab vs. Regeneron show Leronlimab better. Regeneron was advised not to use Regeneron cocktail told to stop using any further high flow oxygen and mechanical ventilation patients into their clinical trial. Something not said and not an exclusion criteria in the Leronlimab trial. That leaves only Leronlimab for severe critical population in a clinical trial. Numbers why don’t you check again about the DSMB. The first look at the severe / critical trial was at 149 patients and that was a safety look. The second look at 195, at 50 % of the 390 designed enrollment , was an efficacy look , with recommendations to take another look at 293 patients . Are you really trying to tell me that wasn’t an efficacy recommendation ? I will ask you the same question I asked Cheese, why do you think they asked for another look at 293 before the full 390 designed to complete the trial? How often does the DSMB recommend another look before the complete trial is over instead of telling the company to add more patients or change their endpoint to succeed in their trial? Like they have done for the other Covid companies trying therapeutics, where they advised to stop the trial for futility, being unsafe, or not going to meet endpoint u less they change the trial !
So we went from “ looking promising” to put on hold... all due respect to the science board gurus. How about we all wait until there is a actual valid break through. This is why people become hyper critical and skeptical. TRUTH... there will be no vaccine at 65-70% ... maybe some alternative interventional meds but a true workable vaccine is years away. This will be an every year AIDS vaccine debacle. How many years? Feel bad for those in the middle age groups.For those that will try to convince us the vaccine or vaccines are here by Spring... better not hold your breath.
You need to read more carefully. They're completely different trials in very different patient populations. Both Regeneron and Lilly are seeing very promising interim data on antibody therapy in mild to moderately ill COVID patients with significant viral load reductions and reduced need for medical interventions. But Lilly's trial in hospitalized patients was stopped due to lack of efficacy and Regeneron's was put on hold for a subset of hospitalized patients (on ventilators and/or high flow O2) while a safety signal is evaluated (similar holds have been placed on the vaccine trials and then rescinded, so we don't know yet, what the issue is), but continues on the rest of the hospitalized patients.
And you're almost certainly wrong about vaccines. SARS-CoV-2 is nothing like the HIV virus with regard to how they replicate, mutate and can be stopped and there is very high confidence that several vaccines will be approved for COVID, with at least 1-2 by the end of the year and several more in 1Q21. We already know they elicit the desired immune responses, but we don't know, yet, how that translates into efficacy and safety over 30K+ patients, which is why we have to do the trials and evaluate them very carefully.
Well, the thread seems to be going ok, so let's try expanding to some other scientific COVID content and see what happens...
An interesting paper was published yesterday on a new genetic variant of the SARS-CoV-2 virus having emerged in Spain and becoming the predominant version of the virus in most infected people in much of Europe since this summer. Right now there's no evidence that this variant is any more or less transmissible or deadly, as that work remains to be done, still. The bolded part is from the first link. The 2nd link is the actual paper.

 www.ft.com
www.ft.com
𝗧𝗵𝗲 𝘀𝗰𝗶𝗲𝗻𝘁𝗶𝗳𝗶𝗰 𝘁𝗲𝗮𝗺𝘀 𝗶𝗻 𝗦𝘄𝗶𝘁𝘇𝗲𝗿𝗹𝗮𝗻𝗱 𝗮𝗻𝗱 𝗦𝗽𝗮𝗶𝗻 𝗮𝗿𝗲 𝗻𝗼𝘄 𝗿𝘂𝘀𝗵𝗶𝗻𝗴 𝘁𝗼 𝗲𝘅𝗮𝗺𝗶𝗻𝗲 𝘁𝗵𝗲 𝗯𝗲𝗵𝗮𝘃𝗶𝗼𝘂𝗿 𝗼𝗳 𝘁𝗵𝗲 𝘃𝗮𝗿𝗶𝗮𝗻𝘁 𝘁𝗼 𝗲𝘀𝘁𝗮𝗯𝗹𝗶𝘀𝗵 𝘄𝗵𝗲𝘁𝗵𝗲𝗿 𝗶𝘁 𝗺𝗮𝘆 𝗯𝗲 𝗺𝗼𝗿𝗲 𝗱𝗲𝗮𝗱𝗹𝘆 𝗼𝗿 𝗺𝗼𝗿𝗲 𝗶𝗻𝗳𝗲𝗰𝘁𝗶𝗼𝘂𝘀 𝘁𝗵𝗮𝗻 𝗼𝘁𝗵𝗲𝗿 𝘀𝘁𝗿𝗮𝗶𝗻𝘀.
𝗗𝗿 𝗛𝗼𝗱𝗰𝗿𝗼𝗳𝘁 𝘀𝘁𝗿𝗲𝘀𝘀𝗲𝗱 𝘁𝗵𝗮𝘁 𝘁𝗵𝗲𝗿𝗲 𝘄𝗮𝘀 “𝗻𝗼 𝗲𝘃𝗶𝗱𝗲𝗻𝗰𝗲 𝘁𝗵𝗮𝘁 𝘁𝗵𝗲 𝘃𝗮𝗿𝗶𝗮𝗻𝘁’𝘀 [𝗿𝗮𝗽𝗶𝗱] 𝘀𝗽𝗿𝗲𝗮𝗱 𝗶𝘀 𝗱𝘂𝗲 𝘁𝗼 𝗮 𝗺𝘂𝘁𝗮𝘁𝗶𝗼𝗻 𝘁𝗵𝗮𝘁 𝗶𝗻𝗰𝗿𝗲𝗮𝘀𝗲𝘀 𝘁𝗿𝗮𝗻𝘀𝗺𝗶𝘀𝘀𝗶𝗼𝗻 𝗼𝗿 𝗶𝗺𝗽𝗮𝗰𝘁𝘀 𝗰𝗹𝗶𝗻𝗶𝗰𝗮𝗹 𝗼𝘂𝘁𝗰𝗼𝗺𝗲”.
https://l.facebook.com/l.php?u=http...kFwA-MblvQucY4q9paltJdrFf9vG2tByKwsGTOKvSY-PA
An interesting paper was published yesterday on a new genetic variant of the SARS-CoV-2 virus having emerged in Spain and becoming the predominant version of the virus in most infected people in much of Europe since this summer. Right now there's no evidence that this variant is any more or less transmissible or deadly, as that work remains to be done, still. The bolded part is from the first link. The 2nd link is the actual paper.

Scientists warn of new coronavirus variant spreading across Europe | Free to read
Genetic mutation that originated in Spain transmitted by returning holidaymakers, researchers find
𝗧𝗵𝗲 𝘀𝗰𝗶𝗲𝗻𝘁𝗶𝗳𝗶𝗰 𝘁𝗲𝗮𝗺𝘀 𝗶𝗻 𝗦𝘄𝗶𝘁𝘇𝗲𝗿𝗹𝗮𝗻𝗱 𝗮𝗻𝗱 𝗦𝗽𝗮𝗶𝗻 𝗮𝗿𝗲 𝗻𝗼𝘄 𝗿𝘂𝘀𝗵𝗶𝗻𝗴 𝘁𝗼 𝗲𝘅𝗮𝗺𝗶𝗻𝗲 𝘁𝗵𝗲 𝗯𝗲𝗵𝗮𝘃𝗶𝗼𝘂𝗿 𝗼𝗳 𝘁𝗵𝗲 𝘃𝗮𝗿𝗶𝗮𝗻𝘁 𝘁𝗼 𝗲𝘀𝘁𝗮𝗯𝗹𝗶𝘀𝗵 𝘄𝗵𝗲𝘁𝗵𝗲𝗿 𝗶𝘁 𝗺𝗮𝘆 𝗯𝗲 𝗺𝗼𝗿𝗲 𝗱𝗲𝗮𝗱𝗹𝘆 𝗼𝗿 𝗺𝗼𝗿𝗲 𝗶𝗻𝗳𝗲𝗰𝘁𝗶𝗼𝘂𝘀 𝘁𝗵𝗮𝗻 𝗼𝘁𝗵𝗲𝗿 𝘀𝘁𝗿𝗮𝗶𝗻𝘀.
𝗗𝗿 𝗛𝗼𝗱𝗰𝗿𝗼𝗳𝘁 𝘀𝘁𝗿𝗲𝘀𝘀𝗲𝗱 𝘁𝗵𝗮𝘁 𝘁𝗵𝗲𝗿𝗲 𝘄𝗮𝘀 “𝗻𝗼 𝗲𝘃𝗶𝗱𝗲𝗻𝗰𝗲 𝘁𝗵𝗮𝘁 𝘁𝗵𝗲 𝘃𝗮𝗿𝗶𝗮𝗻𝘁’𝘀 [𝗿𝗮𝗽𝗶𝗱] 𝘀𝗽𝗿𝗲𝗮𝗱 𝗶𝘀 𝗱𝘂𝗲 𝘁𝗼 𝗮 𝗺𝘂𝘁𝗮𝘁𝗶𝗼𝗻 𝘁𝗵𝗮𝘁 𝗶𝗻𝗰𝗿𝗲𝗮𝘀𝗲𝘀 𝘁𝗿𝗮𝗻𝘀𝗺𝗶𝘀𝘀𝗶𝗼𝗻 𝗼𝗿 𝗶𝗺𝗽𝗮𝗰𝘁𝘀 𝗰𝗹𝗶𝗻𝗶𝗰𝗮𝗹 𝗼𝘂𝘁𝗰𝗼𝗺𝗲”.
https://l.facebook.com/l.php?u=http...kFwA-MblvQucY4q9paltJdrFf9vG2tByKwsGTOKvSY-PA
That is my point. Only the DSMB has seen the actual data . You cannot come out and say their recommendation to exclude on high flow oxygen and ventilators and halting for safety concerns is good news for Regeneron cocktail and boast that it will be better than all the others. I will agree with you that it will be better than most of the others that have failed in their trials but definitely not better than Leronlimab which trial was not stopped for safety nor for efficacy and no one was excluded. I know randomized trials are the gold standard and Cytodyn completed one on M2M and is ongoing on S/C but the reason the FDA approves the trials is because Leronlimab was given by EIND to about 60-65 patients in really bad shape and it got most off of echmo, patients on kidney dialysis and really sick people with very few deaths.There is a DMSB for every trial, so nobody has compared the Regeneron antibody cocktail vs. Leronlimab. Leronlimab may end up being safer for seriously ill, hospitalized patients, but we don't know that yet (the Regeneron trial was put on "hold" in patients on ventilators or with high flow O2, not stopped, so we don't know if it will continue). And trials at an interim point are occasionally asked by DMSB's to look at a further interim point downstream before the endpoint - neither you nor I "know" why they did that in Leronlimab's case (only the DSMB is unblinded to the data).
More good news today, Dr. Bruce Patterson ‘s manuscript describing the MOA of CoVid and it being a Rantes disease was approved for publication. Patterson did the assays of the EIND patients for Leronlimab and saw how all the critical markers, Ig6, T cell, etc. improved drastically by Day 3 after the use of Leronlimab.
One last point , the M2M trial completed by Cytodyn showed improvement by Day 3 after use of Leronlimab but because trial size was small( 56 Leronlimab, 28 placebo) and people get better on their own by 14 days , FDA did not give approval and advised company to proceed with S/C trial. No reason Leronlimab not approved for M2M as an EUA when Remdesiver or Regeneron have or will get approved.
What I am saying specifically is we all want the same thing numbers. We all pray... well maybe not all ... that the scientific field will once again give us that miracle type drug or therapeutic... please understand this was not intended to offend you or any of our brilliant in the med / sci world. I don’t claim to know anywhere near what those trained and educated do. I would think perhaps 95% of the world’s population even the highly educated don’t truly realize the difficulty nor the walls which have prevented more success. I think sometime as interesting and hopeful the information is it lends itself to getting up false hopes. Here’s another issue raised in Europe again regarding masks. Germany and Spain are 95% in lock step with face coverings yet their numbers are as bad as ours and perhaps worse. Several of the TOP virologists working on Covid are skeptical again as to actually what the masks do or don’t do? They may help but perhaps it really does not matter. Keep the info coming and of course our Rutgers football sports news. Did not mean to cause another uproar...You need to read more carefully. They're completely different trials in very different patient populations. Both Regeneron and Lilly are seeing very promising interim data on antibody therapy in mild to moderately ill COVID patients with significant viral load reductions and reduced need for medical interventions. But Lilly's trial in hospitalized patients was stopped due to lack of efficacy and Regeneron's was put on hold for a subset of hospitalized patients (on ventilators and/or high flow O2) while a safety signal is evaluated (similar holds have been placed on the vaccine trials and then rescinded, so we don't know yet, what the issue is), but continues on the rest of the hospitalized patients.
And you're almost certainly wrong about vaccines. SARS-CoV-2 is nothing like the HIV virus with regard to how they replicate, mutate and can be stopped and there is very high confidence that several vaccines will be approved for COVID, with at least 1-2 by the end of the year and several more in 1Q21. We already know they elicit the desired immune responses, but we don't know, yet, how that translates into efficacy and safety over 30K+ patients, which is why we have to do the trials and evaluate them very carefully.
I never said the DMSB recommendation for Regeneron to hold on dosing patients on vents and/or under high O2 flow was "good news." I have zero idea why you'd say I said that. The very promising Regeneron (and Lilly) interim clinical data are on mild to moderately ill COVID patients prior to hospitalization. Totally different trials/patients.That is my point. Only the DSMB has seen the actual data . You cannot come out and say their recommendation to exclude on high flow oxygen and ventilators and halting for safety concerns is good news for Regeneron cocktail and boast that it will be better than all the others. I will agree with you that it will be better than most of the others that have failed in their trials but definitely not better than Leronlimab which trial was not stopped for safety nor for efficacy and no one was excluded. I know randomized trials are the gold standard and Cytodyn completed one on M2M and is ongoing on S/C but the reason the FDA approves the trials is because Leronlimab was given by EIND to about 60-65 patients in really bad shape and it got most off of echmo, patients on kidney dialysis and really sick people with very few deaths.
More good news today, Dr. Bruce Patterson ‘s manuscript describing the MOA of CoVid and it being a Rantes disease was approved for publication. Patterson did the assays of the EIND patients for Leronlimab and saw how all the critical markers, Ig6, T cell, etc. improved drastically by Day 3 after the use of Leronlimab.
One last point , the M2M trial completed by Cytodyn showed improvement by Day 3 after use of Leronlimab but because trial size was small( 56 Leronlimab, 28 placebo) and people get better on their own by 14 days , FDA did not give approval and advised company to proceed with S/C trial. No reason Leronlimab not approved for M2M as an EUA when Remdesiver or Regeneron have or will get approved.
I'm also saying we don't know why the leronlimab DMSB asked for an additional review point, but you're saying you "know" it's because the clinical data so far are good. Which is flat out wrong and you know it. That could be the case, but you absolutely don't "know" what only the DMSB knows. You really need to be more careful with your wording of things.
RU848789 - just want to say "thank you" for your generous sharing of this information on the antibody cocktails. Much appreciated!!! And thank you to the RU football community for not making this political in any way.
Let’s try 1 more time. Why in your opinion would the DSMB ask Cytodyn to take another look at when the trial is 75% enrolled at 293 patients instead of just say wait until trial is over after 390 patients ? It you think that doesn’t strongly infer efficacy and likely when they think statistical significance will be found, then you are being dishonest since the DSMB made the recommendation not the company and hey are the only ones to see the actual data.I never said the DMSB recommendation for Regeneron to hold on dosing patients on vents and/or under high O2 flow was "good news." I have zero idea why you'd say I said that. The very promising Regeneron (and Lilly) interim clinical data are on mild to moderately ill COVID patients prior to hospitalization. Totally different trials/patients.
I'm also saying we don't know why the leronlimab DMSB asked for an additional review point, but you're saying you "know" it's because the clinical data so far are good. Which is flat out wrong and you know it. That could be the case, but you absolutely don't "know" what only the DMSB knows. You really need to be more careful with your wording of things.
There are at least two other reasons to ask for an earlier interim look at the data. First, perhaps there was a hint of some safety problem, although not enough to stop the trial, but the DMSB wanted to get an earlier read in case there was a real safety issue, so they could protect patient safety. Second, it's possible that there was a weak efficacy signal - just enough to keep going (and not stopping the trial), but the DMSB wanted an earlier read before the end, in case that showed clear non-efficacy earlier, so they could stop the trial and not waste the time/effort in finishing it. But neither of us knows why and there could be other reasons.Let’s try 1 more time. Why in your opinion would the DSMB ask Cytodyn to take another look at when the trial is 75% enrolled at 293 patients instead of just say wait until trial is over after 390 patients ? It you think that doesn’t strongly infer efficacy and likely when they think statistical significance will be found, then you are being dishonest since the DSMB made the recommendation not the company and hey are the only ones to see the actual data.
Also, it's "imply" not "infer."
Thanks for at least answering but you are lost. Leronlimab has had no safety issues in over 1000 patients over multiple years as it was being readied to be approved for AIDS where trials have been completed. There were no safety issues in the 60-70 EIND patients, no safety issues in the M2M completed trial and no safety issues when the first interim look was taken by the DSMB on the severe trial at 149 patients. Leronlimab is the safest drug out there that would treat CoVid and multiple other indications. Your second guess is mind numbing . By telling Cytodyn to continue the trial without changing the endpoint with no changes, and not to add patients but rather let’s look again at 75% enrollment , the most probable explaination if you were being honest, is that the data was showing clinical significance but not statistical significance just yet. The DSMB is taking turns on all of Big Pharma drugs halting trials for safety, Eli Lilly’s , Gilead’s, and now Regeneron for some oxygenated and ventilated patients, as well as stopping trials due to futility for many others, like the arthritis drug , Kevzara. You know which drug has not had any of that happen during its trials , that’s right it’s LeronlimabThere are at least two other reasons to ask for an earlier interim look at the data. First, perhaps there was a hint of some safety problem, although not enough to stop the trial, but the DMSB wanted to get an earlier read in case there was a real safety issue, so they could protect patient safety. Second, it's possible that there was a weak efficacy signal - just enough to keep going (and not stopping the trial), but the DMSB wanted an earlier read before the end, in case that showed clear non-efficacy earlier, so they could stop the trial and not waste the time/effort in finishing it. But neither of us knows why and there could be other reasons.
Also, it's "imply" not "infer."
Thanks for at least answering but you are lost. Leronlimab has had no safety issues in over 1000 patients over multiple years as it was being readied to be approved for AIDS where trials have been completed. There were no safety issues in the 60-70 EIND patients, no safety issues in the M2M completed trial and no safety issues when the first interim look was taken by the DSMB on the severe trial at 149 patients. Leronlimab is the safest drug out there that would treat CoVid and multiple other indications. Your second guess is mind numbing . By telling Cytodyn to continue the trial without changing the endpoint with no changes, and not to add patients but rather let’s look again at 75% enrollment , the most probable explaination if you were being honest, is that the data was showing clinical significance but not statistical significance just yet. The DSMB is taking turns on all of Big Pharma drugs halting trials for safety, Eli Lilly’s , Gilead’s, and now Regeneron for some oxygenated and ventilated patients, as well as stopping trials due to futility for many others, like the arthritis drug , Kevzara. You know which drug has not had any of that happen during its trials , that’s right it’s Leronlimab
Safety in those other trials is far less meaningful with regard to safety in COVID patients, which is a completely different disease. At the end of the day neither one of us has any idea why the DSMB wants to look at an earlier interim timepoint in the trial. Your track record, to date, has been poor on this, and you don't even understand statistical significance and p-values, so forgive me if I'm not inclined to agree with your take on this. I suggest we wait to find out what happens over the rest of the trial and stop wasting everyone else's time in this thread.
@goru7 Just curious but what is your relationship with CYDY? Are you an investor, do you work there, etc.? Your posts seem to carry lot of emotion behind them so I'm curious.Safety in those other trials is far less meaningful with regard to safety in COVID patients, which is a completely different disease. At the end of the day neither one of us has any idea why the DSMB wants to look at an earlier interim timepoint in the trial. Your track record, to date, has been poor on this, and you don't even understand statistical significance and p-values, so forgive me if I'm not inclined to agree with your take on this. I suggest we wait to find out what happens over the rest of the trial and stop wasting everyone else's time in this thread.
The mechanism of action is that Leronlimab blocks the CCR5 receptor and prevents the virus from replicating and causing the storm . It has worked on mild to moderate patients and is working on severe / critical patients. If the CEO did not make missteps along the way, the results would be completed by now. But because Leronlimab has indications to treat AIDS, triple breast cancer, Nash, Gvhd, and other indications, The FDA and its former Gilead employees have not made it easy to get approval as it will be a huge competitor for many indications. Once approval happens whether it t be in the USA or in the U.K. or Eu , the other approvals will follow. It will be approved for AIDS in 6-9 months and likely sooner in the U.K. and Canada
CCR5 is receptor for HIV. CCR5 is not a receptor for SARS-Cov-2.
Tried that and other angles already - he's obviously either a rep for them or has a ton of $$ invested with them - they're the only logical explanations...CCR5 is receptor for HIV. CCR5 is not a receptor for SARS-Cov-2.
It is . It is the MOA. Read Dr. Bruce Patterson’s manuscript. Explains it allCCR5 is receptor for HIV. CCR5 is not a receptor for SARS-Cov-2.
You are an asshat. Get educated. Read Patterson’s manuscript. Covid is a Rantes disease and Leronlimab blocks it at the CCR5 receptor . But it also decreases viral load and brings your immune system To be in homo Statis and works on its own. Unlike the other drugs that only reduce Ig6 have not proven effective to date. Leronlimab does more.Tried that and other angles already - he's obviously either a rep for them or has a ton of $$ invested with them - they're the only logical explanations...
It was in trials for HIV for years and they realized it might work on Covid. Once the MOA is understood it makes sense logically and scientifically.CCR5 is receptor for HIV. CCR5 is not a receptor for SARS-Cov-2.
It is . It is the MOA. Read Dr. Bruce Patterson’s manuscript. Explains it all
so all the work and crystal structures and so on that show that ACE2 is the receptor are wrong? What’s the full reference for the Patterson paper?
https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa1583/5932277 This is a recent paper published by Dr. Yang who treated the EIND patients. Trying to locate Patterson’s link. Here it https://www.medrxiv.org/content/10.1101/2020.05.02.20084673v1so all the work and crystal structures and so on that show that ACE2 is the receptor are wrong? What’s the full reference for the Patterson paper?
so all the work and crystal structures and so on that show that ACE2 is the receptor are wrong? What’s the full reference for the Patterson paper?
Of course not, as I know you know. I'm also guessing you saw the recent breakthroughs on cryogenic electron microscopy and its application to being able to essentially "see" down to just about the molecular level for the proteins, potentially obviating the need to grow pure single crystals of proteins and even small molecules in order to determine structure, which historically has been done via X-ray diffraction patterns on such crystals. Derek Lowe posted on this yesterday on his blog, linked below, as two breakthrough papers on this technology were just published in Nature.
Crystals are very precisely ordered/arranged and the diffraction pattern can be analyzed via software to determine exactly how molecules are connected and crystals are usually fairly easy to isolate for small molecules (although not always - I've done a ton of work in the field of crystallography and have 2 patents in this area). However, it's often extremely hard to grow pure crystals of biological molecules like proteins, so their structure can be elucidated by X-rays. But with cryo electron microscopy, it may not always be necessary any more as this technology can elucidate 3-D structure directly (via very advanced software).
https://blogs.sciencemag.org/pipeline/archives/2020/10/30/down-to-the-atoms
https://www.nature.com/articles/s41586-020-2829-0
https://www.nature.com/articles/s41586-020-2833-4
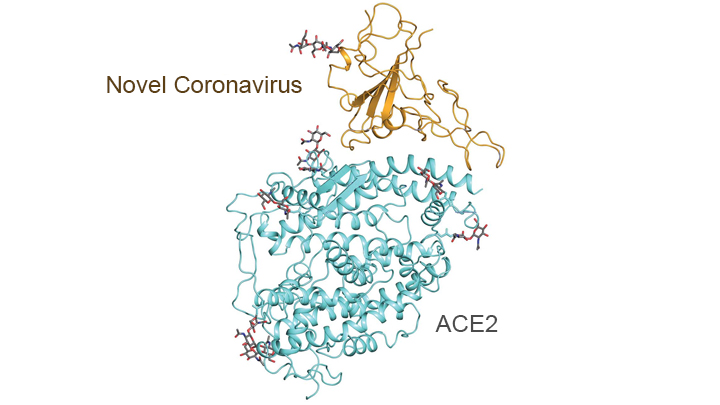
In addition, this technology has been put to work to help elucidate some of the structural elements of the proteins on the SARS-CoV-2 virus and how they interact with various human proteins - in particular the peptidases of the angiotensin-converting enzyme 2 (ACE2), which the virus "mates with" via its spike proteins to gain entry into various epithelial cells in the lungs and blood vessels and elsewhere. The graphic below shows the outcome of some of this work - pretty cool stuff.
https://directorsblog.nih.gov/tag/cryo-em/
You completely flubbed the discussion of statistical clinical significance the other day, you've been overpromising on leronlimab for months, you seem to think CCR5 is associated with SARS-CoV-2, and now you just butchered this post (it's homeostasis, not "homo Statis" - don't even want to know what that is) and I'm the asshat? Also, that "paper" you keep citing is a preprint from May and hasn't actually been published in reputable scientific journal yet.You are an asshat. Get educated. Read Patterson’s manuscript. Covid is a Rantes disease and Leronlimab blocks it at the CCR5 receptor . But it also decreases viral load and brings your immune system To be in homo Statis and works on its own. Unlike the other drugs that only reduce Ig6 have not proven effective to date. Leronlimab does more.
While a CCR5 antagonist, like leronlimab, may well have efficacy in reducing the inflammation cascade that leads to serious illness and even death in some COVID patients, the molecule has no direct effect on the virus (and shouldn't on viral loads) and we've also yet to see any efficacy data on leronlimab from any controlled, randomized clinical trials - we've only seen some anecdotal observational results, which looked "promising." If you end up being "right" about leronlimab being efficacious, it'll be the equivalent of a broken clock being right twice a day - it won't be because you had any medical/scientific understanding or insight. I continue to hope that the drug is effective, as I'm sure everyone does.
Post below and the two papers linked in it explain what an epitope is on the virus's spike protein receptor binding domain in great detail, but basically, an epitope is simply the part of an antigen (foreign body, like a virus) that is recognized by some part of the immune system, such as antibodies, B-cells or T-cells - and monoclonal antibodies are "created" to behave just like human-generated antibodies and augment our immune system, since many people don't make enough of these antibodies.
https://en.wikipedia.org/wiki/Epitope
https://rutgers.forums.rivals.com/t...es-interventions-and-more.191275/post-4609892
#'s...are there any studies using CRiSPR?
If so how succsessful have they been?
if not: why?
Thanks!
MO
This is concerning. Hopefully some of these treatments will mitigate this
Israel vaccine trials
https://www.google.com/amp/s/www.fo...gins-human-trials-for-coronavirus-vaccine.amp
https://www.google.com/amp/s/www.fo...gins-human-trials-for-coronavirus-vaccine.amp
You completely flubbed the discussion of statistical clinical significance the other day, you've been overpromising on leronlimab for months, you seem to think CCR5 is associated with SARS-CoV-2, and now you just butchered this post (it's homeostasis, not "homo Statis" - don't even want to know what that is) and I'm the asshat? Also, that "paper" you keep citing is a preprint from May and hasn't actually been published in reputable scientific journal yet.
While a CCR5 antagonist, like leronlimab, may well have efficacy in reducing the inflammation cascade that leads to serious illness and even death in some COVID patients, the molecule has no direct effect on the virus (and shouldn't on viral loads) and we've also yet to see any efficacy data on leronlimab from any controlled, randomized clinical trials - we've only seen some anecdotal observational results, which looked "promising." If you end up being "right" about leronlimab being efficacious, it'll be the equivalent of a broken clock being right twice a day - it won't be because you had any medical/scientific understanding or insight. I continue to hope that the drug is effective, as I'm sure everyone does.
I've been following several covid treatments over the last 6 months. I have never seen retail shareholders pumping a stock like they have for Cytodyn. Shareholders have been relentlessly spamming every social media platform and government twitter account with the same press releases and articles and now this paper from their cult leader, Bruce Paterson. Search $CYDY on twitter. It's a circus.
I know, I've seen it and we discussed it in the old COVID thread. I'm not going to say the drug isn't going to work, because it might, but the market hypesters are extremely aggressive on this one.I've been following several covid treatments over the last 6 months. I have never seen retail shareholders pumping a stock like they have for Cytodyn. Shareholders have been relentlessly spamming every social media platform and government twitter account with the same press releases and articles and now this paper from their cult leader, Bruce Paterson. Search $CYDY on twitter. It's a circus.
Y
Please advise one doctor that says it has not worked ? Do you own research and figure out how many doctors, including those on the front line , that comment about how it works! If it wasn’t a small company, and if their results were produced by Big Pharma , they would have been approved 2 months ago when the M2M trial was completed. While you are doing your research, find me another company that has gone through 2 double blinded placebo controlled 2:1, controlled trials that has been found safe, and not had their trials halted for safety, or futility like all the others or asked to change their endpoint or modify their trials . Looking forward to what your research reveals, and whether you believe it should be approved for At least EUA !I've been following several covid treatments over the last 6 months. I have never seen retail shareholders pumping a stock like they have for Cytodyn. Shareholders have been relentlessly spamming every social media platform and government twitter account with the same press releases and articles and now this paper from their cult leader, Bruce Paterson. Search $CYDY on twitter. It's a circus.
He sounds like a rep.@goru7 Just curious but what is your relationship with CYDY? Are you an investor, do you work there, etc.? Your posts seem to carry lot of emotion behind them so I'm curious.
Will try to repost some interesting scientific findings related to COVID that I've posted elsewhere recently, but not on this board as there wasn't anywhere to post them. Hopefully we can keep this as a non-political COVID science thread, especially since we're just about done with the election and coronavirus doesn't pay attention to elections.
Anyway, about 2 weeks ago we got some disappointing results from the WHO's large scale "Solidarity" set of clinical trials evaluating repurposed drugs, including remdesivir, HCQ, the lopinavir/ritonavir antiviral combination, and interferon-beta1 (with and without lopinavir) with COVID-19, with none of them showing any efficacy.
The only real surprise here is remdesivir not showing efficacy, although its efficacy in the NIH trial was not particularly strong (but still significant in reducing hospitalization time) and different clinical trials can show different results, when efficacy is borderline. Here's how Derek Lowe (yep, that guy again, but he's that good) from ScienceMag's "In the Pipeline" blog put it.
𝐼 𝑡ℎ𝑖𝑛𝑘 𝑡ℎ𝑎𝑡’𝑠 𝑡ℎ𝑒 𝑚𝑎𝑗𝑜𝑟 𝑡𝑎𝑘𝑒-ℎ𝑜𝑚𝑒. 𝐼𝑡 𝑙𝑜𝑜𝑘𝑠 𝑙𝑖𝑘𝑒 𝑡ℎ𝑒𝑟𝑒 𝑖𝑠 𝑛𝑜 𝑐𝑎𝑠𝑒 𝑎𝑡 𝑎𝑙𝑙 𝑡𝑜 𝑏𝑒 𝑚𝑎𝑑𝑒 𝑓𝑜𝑟 𝑠𝑜𝑚𝑒 𝑜𝑓 𝑡ℎ𝑒𝑠𝑒 𝑡ℎ𝑒𝑟𝑎𝑝𝑖𝑒𝑠, 𝑎𝑛𝑑 𝑓𝑜𝑟 𝑟𝑒𝑚𝑑𝑒𝑠𝑖𝑣𝑖𝑟, 𝑖𝑡 𝑙𝑜𝑜𝑘𝑠 𝑙𝑖𝑘𝑒 𝑡ℎ𝑒 𝑎𝑟𝑔𝑢𝑚𝑒𝑛𝑡 𝑛𝑜𝑤 𝑖𝑠 “𝐷𝑜𝑒𝑠 𝑖𝑡 ℎ𝑒𝑙𝑝 𝑎 𝑏𝑖𝑡 𝑜𝑟 𝑗𝑢𝑠𝑡 𝑛𝑜𝑡 𝑎𝑡 𝑎𝑙𝑙?” 𝑇ℎ𝑒 𝑜𝑛𝑙𝑦 𝑡ℎ𝑖𝑛𝑔 𝑡ℎ𝑎𝑡 𝐼’𝑣𝑒 𝑠𝑒𝑒𝑛 𝑡ℎ𝑎𝑡 𝑟𝑒𝑎𝑙𝑙𝑦 𝑠𝑒𝑒𝑚𝑠 𝑡𝑜 𝑏𝑒 𝑚𝑎𝑘𝑖𝑛𝑔 𝑎 𝑏𝑖𝑔 𝑑𝑖𝑓𝑓𝑒𝑟𝑒𝑛𝑐𝑒 𝑛𝑜𝑤 𝑖𝑠 𝑑𝑒𝑥𝑎𝑚𝑒𝑡ℎ𝑎𝑠𝑜𝑛𝑒, 𝑎𝑛𝑑 𝑤𝑒 𝑚𝑎𝑦 𝑠𝑜𝑜𝑛 (𝐼 ℎ𝑜𝑝𝑒) 𝑏𝑒 𝑎𝑑𝑑𝑖𝑛𝑔 𝑡ℎ𝑒 𝑚𝑜𝑛𝑜𝑐𝑙𝑜𝑛𝑎𝑙 𝑎𝑛𝑡𝑖𝑏𝑜𝑑𝑖𝑒𝑠 𝑡𝑜 𝑡ℎ𝑎𝑡 𝑙𝑖𝑠𝑡. 𝑇ℎ𝑒 𝑆𝑂𝐿𝐼𝐷𝐴𝑅𝐼𝑇𝑌 𝑒𝑛𝑟𝑜𝑙𝑙𝑚𝑒𝑛𝑡 𝑖𝑠 𝑠𝑡𝑖𝑙𝑙 𝑚𝑜𝑣𝑖𝑛𝑔 𝑎𝑙𝑜𝑛𝑔 𝑎𝑡 2000 𝑝𝑎𝑡𝑖𝑒𝑛𝑡𝑠/𝑚𝑜𝑛𝑡ℎ, 𝑎𝑛𝑑 𝑤𝑖𝑙𝑙 𝑏𝑒 𝑙𝑜𝑜𝑘𝑖𝑛𝑔 𝑎𝑡 𝑡ℎ𝑒𝑠𝑒 𝑎𝑛𝑑 𝑜𝑡ℎ𝑒𝑟 𝑛𝑒𝑤𝑒𝑟 𝑖𝑑𝑒𝑎𝑠 𝑎𝑠 𝑖𝑡 𝑐𝑜𝑛𝑡𝑖𝑛𝑢𝑒𝑠. 𝐴𝑛𝑑 𝑡ℎ𝑎𝑡 𝑚𝑒𝑎𝑛𝑠 𝑎𝑏𝑎𝑛𝑑𝑜𝑛𝑖𝑛𝑔 𝑡ℎ𝑒 𝑜𝑙𝑑𝑒𝑟 𝑖𝑑𝑒𝑎𝑠, 𝑏𝑒𝑐𝑎𝑢𝑠𝑒 – 𝑎𝑠 𝑤𝑒’𝑟𝑒 𝑓𝑖𝑛𝑑𝑖𝑛𝑔 𝑜𝑢𝑡 – 𝑡ℎ𝑒𝑦’𝑟𝑒 𝑛𝑜𝑡 𝑚𝑢𝑐ℎ ℎ𝑒𝑙𝑝. 𝐼𝑡’𝑠 𝑡𝑖𝑚𝑒 𝑡𝑜 𝑚𝑜𝑣𝑒 𝑜𝑛.
https://blogs.sciencemag.org/pipeline/archives/2020/10/16/the-solidarity-data
https://www.medrxiv.org/content/10.1101/2020.10.15.20209817v1.full.pdf
Another article in Science was much harsher on Gilead, the company that developed remdesivir and has been driving its COVID clinical trials, as well as on the FDA and EMA (European Medicines Agency) for approving remdesivir without considering the negative results from the Solidarity trial.
https://www.sciencemag.org/news/202...gbL7Ur4EfuKiGtsfenmIqcfpXYFQcOkSCkSot19sy1MaY
Anyway, about 2 weeks ago we got some disappointing results from the WHO's large scale "Solidarity" set of clinical trials evaluating repurposed drugs, including remdesivir, HCQ, the lopinavir/ritonavir antiviral combination, and interferon-beta1 (with and without lopinavir) with COVID-19, with none of them showing any efficacy.
The only real surprise here is remdesivir not showing efficacy, although its efficacy in the NIH trial was not particularly strong (but still significant in reducing hospitalization time) and different clinical trials can show different results, when efficacy is borderline. Here's how Derek Lowe (yep, that guy again, but he's that good) from ScienceMag's "In the Pipeline" blog put it.
𝐼 𝑡ℎ𝑖𝑛𝑘 𝑡ℎ𝑎𝑡’𝑠 𝑡ℎ𝑒 𝑚𝑎𝑗𝑜𝑟 𝑡𝑎𝑘𝑒-ℎ𝑜𝑚𝑒. 𝐼𝑡 𝑙𝑜𝑜𝑘𝑠 𝑙𝑖𝑘𝑒 𝑡ℎ𝑒𝑟𝑒 𝑖𝑠 𝑛𝑜 𝑐𝑎𝑠𝑒 𝑎𝑡 𝑎𝑙𝑙 𝑡𝑜 𝑏𝑒 𝑚𝑎𝑑𝑒 𝑓𝑜𝑟 𝑠𝑜𝑚𝑒 𝑜𝑓 𝑡ℎ𝑒𝑠𝑒 𝑡ℎ𝑒𝑟𝑎𝑝𝑖𝑒𝑠, 𝑎𝑛𝑑 𝑓𝑜𝑟 𝑟𝑒𝑚𝑑𝑒𝑠𝑖𝑣𝑖𝑟, 𝑖𝑡 𝑙𝑜𝑜𝑘𝑠 𝑙𝑖𝑘𝑒 𝑡ℎ𝑒 𝑎𝑟𝑔𝑢𝑚𝑒𝑛𝑡 𝑛𝑜𝑤 𝑖𝑠 “𝐷𝑜𝑒𝑠 𝑖𝑡 ℎ𝑒𝑙𝑝 𝑎 𝑏𝑖𝑡 𝑜𝑟 𝑗𝑢𝑠𝑡 𝑛𝑜𝑡 𝑎𝑡 𝑎𝑙𝑙?” 𝑇ℎ𝑒 𝑜𝑛𝑙𝑦 𝑡ℎ𝑖𝑛𝑔 𝑡ℎ𝑎𝑡 𝐼’𝑣𝑒 𝑠𝑒𝑒𝑛 𝑡ℎ𝑎𝑡 𝑟𝑒𝑎𝑙𝑙𝑦 𝑠𝑒𝑒𝑚𝑠 𝑡𝑜 𝑏𝑒 𝑚𝑎𝑘𝑖𝑛𝑔 𝑎 𝑏𝑖𝑔 𝑑𝑖𝑓𝑓𝑒𝑟𝑒𝑛𝑐𝑒 𝑛𝑜𝑤 𝑖𝑠 𝑑𝑒𝑥𝑎𝑚𝑒𝑡ℎ𝑎𝑠𝑜𝑛𝑒, 𝑎𝑛𝑑 𝑤𝑒 𝑚𝑎𝑦 𝑠𝑜𝑜𝑛 (𝐼 ℎ𝑜𝑝𝑒) 𝑏𝑒 𝑎𝑑𝑑𝑖𝑛𝑔 𝑡ℎ𝑒 𝑚𝑜𝑛𝑜𝑐𝑙𝑜𝑛𝑎𝑙 𝑎𝑛𝑡𝑖𝑏𝑜𝑑𝑖𝑒𝑠 𝑡𝑜 𝑡ℎ𝑎𝑡 𝑙𝑖𝑠𝑡. 𝑇ℎ𝑒 𝑆𝑂𝐿𝐼𝐷𝐴𝑅𝐼𝑇𝑌 𝑒𝑛𝑟𝑜𝑙𝑙𝑚𝑒𝑛𝑡 𝑖𝑠 𝑠𝑡𝑖𝑙𝑙 𝑚𝑜𝑣𝑖𝑛𝑔 𝑎𝑙𝑜𝑛𝑔 𝑎𝑡 2000 𝑝𝑎𝑡𝑖𝑒𝑛𝑡𝑠/𝑚𝑜𝑛𝑡ℎ, 𝑎𝑛𝑑 𝑤𝑖𝑙𝑙 𝑏𝑒 𝑙𝑜𝑜𝑘𝑖𝑛𝑔 𝑎𝑡 𝑡ℎ𝑒𝑠𝑒 𝑎𝑛𝑑 𝑜𝑡ℎ𝑒𝑟 𝑛𝑒𝑤𝑒𝑟 𝑖𝑑𝑒𝑎𝑠 𝑎𝑠 𝑖𝑡 𝑐𝑜𝑛𝑡𝑖𝑛𝑢𝑒𝑠. 𝐴𝑛𝑑 𝑡ℎ𝑎𝑡 𝑚𝑒𝑎𝑛𝑠 𝑎𝑏𝑎𝑛𝑑𝑜𝑛𝑖𝑛𝑔 𝑡ℎ𝑒 𝑜𝑙𝑑𝑒𝑟 𝑖𝑑𝑒𝑎𝑠, 𝑏𝑒𝑐𝑎𝑢𝑠𝑒 – 𝑎𝑠 𝑤𝑒’𝑟𝑒 𝑓𝑖𝑛𝑑𝑖𝑛𝑔 𝑜𝑢𝑡 – 𝑡ℎ𝑒𝑦’𝑟𝑒 𝑛𝑜𝑡 𝑚𝑢𝑐ℎ ℎ𝑒𝑙𝑝. 𝐼𝑡’𝑠 𝑡𝑖𝑚𝑒 𝑡𝑜 𝑚𝑜𝑣𝑒 𝑜𝑛.
https://blogs.sciencemag.org/pipeline/archives/2020/10/16/the-solidarity-data
https://www.medrxiv.org/content/10.1101/2020.10.15.20209817v1.full.pdf
Another article in Science was much harsher on Gilead, the company that developed remdesivir and has been driving its COVID clinical trials, as well as on the FDA and EMA (European Medicines Agency) for approving remdesivir without considering the negative results from the Solidarity trial.
https://www.sciencemag.org/news/202...gbL7Ur4EfuKiGtsfenmIqcfpXYFQcOkSCkSot19sy1MaY
In case folks didn't see this, here is the good news on the vaccine trial holds being lifted on the AstraZeneca and J&J US phase 3 vaccine clinical trials for Covid-19. AZ's trial was put on hold soon after it began in early September, following news of a UK participant who developed a transverse myelitis (spinal cord inflammation) after receiving the vaccine, while J&J's trial was paused on Oct. 12 after an unspecified injury in one of its study participants. We certainly need as many options as we can get, since none of the vaccines is guaranteed to be effective, plus getting vaccines to as many people as possible, as quickly as we can is important and having more available will help, both here and abroad.
https://www.necn.com/news/coronavir...e-late-stage-covid-19-vaccine-trials/2338936/
https://www.necn.com/news/coronavir...e-late-stage-covid-19-vaccine-trials/2338936/
- Status
- Not open for further replies.
Similar threads
ADVERTISEMENT
Latest posts
-
***Ask The Experts Presented by American Financial Network’s Greg Ginn***
- Latest: rutgersspeak95
-
-
***WAR ROOM: Latest Recruit Scoop and Team News on Rutgers Athletics***
- Latest: Arizona Knight
-
ADVERTISEMENT

