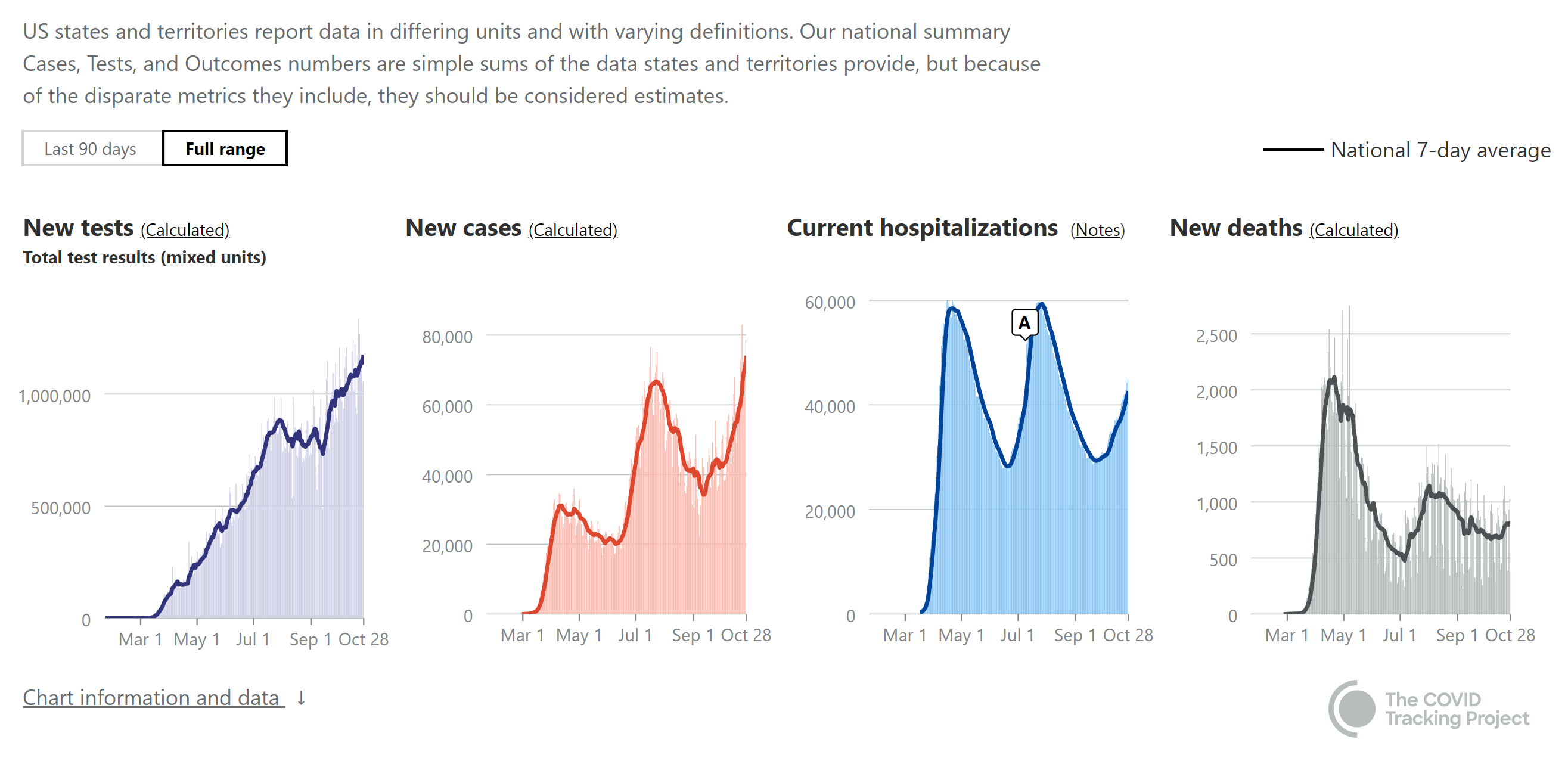
My bet since March was that Regeneron's antibody cocktail (mix of two monoclonal antibodies that target two different epitopes on the virus's spike protein) was going to be our best hope for seriously improving patient response to being infected and that seems like what we're seeing with today's additional results from the ongoing phase II/III trial in mild to moderately ill COVID patients (prior to hospitalization, when an antiviral is likely to have the biggest benefit).
Certainly can't say it's a "cure" yet, but it's looking much better than any other drug out there, so far and could be a bridge to vaccines, especially as it's also being looked at as a prophylactic to prevent infection in high risk workers/possible patients. Regeneron has applied with the FDA for an Emergency Use Authorization soon, which I think makes sense. Yeah, I know - no COVID threads, but people ought to know about this. At least leave it up for a day or so. This is really important news given that Eli Lilly announced that they were stopping their trial in hospitalized COVID patients getting their single antibody treatment along with remdesivir, due to lack of efficacy (they also have a cocktail - awaiting results on that).
https://investor.regeneron.com/news...9-outpatient-trial-prospectively-demonstrates
𝗥𝗲𝗴𝗲𝗻𝗲𝗿𝗼𝗻 𝗣𝗵𝗮𝗿𝗺𝗮𝗰𝗲𝘂𝘁𝗶𝗰𝗮𝗹𝘀, 𝗜𝗻𝗰. (𝗡𝗔𝗦𝗗𝗔𝗤: 𝗥𝗘𝗚𝗡) 𝘁𝗼𝗱𝗮𝘆 𝗮𝗻𝗻𝗼𝘂𝗻𝗰𝗲𝗱 𝗽𝗼𝘀𝗶𝘁𝗶𝘃𝗲, 𝗽𝗿𝗼𝘀𝗽𝗲𝗰𝘁𝗶𝘃𝗲 𝗿𝗲𝘀𝘂𝗹𝘁𝘀 𝗳𝗿𝗼𝗺 𝗮𝗻 𝗼𝗻𝗴𝗼𝗶𝗻𝗴 𝗣𝗵𝗮𝘀𝗲 𝟮/𝟯 𝘀𝗲𝗮𝗺𝗹𝗲𝘀𝘀 𝘁𝗿𝗶𝗮𝗹 𝗶𝗻 𝘁𝗵𝗲 𝗖𝗢𝗩𝗜𝗗-𝟭𝟵 𝗼𝘂𝘁𝗽𝗮𝘁𝗶𝗲𝗻𝘁 𝘀𝗲𝘁𝘁𝗶𝗻𝗴 𝘀𝗵𝗼𝘄𝗶𝗻𝗴 𝗶𝘁𝘀 𝗶𝗻𝘃𝗲𝘀𝘁𝗶𝗴𝗮𝘁𝗶𝗼𝗻𝗮𝗹 𝗮𝗻𝘁𝗶𝗯𝗼𝗱𝘆 𝗰𝗼𝗰𝗸𝘁𝗮𝗶𝗹, 𝗥𝗘𝗚𝗡-𝗖𝗢𝗩𝟮, 𝗺𝗲𝘁 𝘁𝗵𝗲 𝗽𝗿𝗶𝗺𝗮𝗿𝘆 𝗮𝗻𝗱 𝗸𝗲𝘆 𝘀𝗲𝗰𝗼𝗻𝗱𝗮𝗿𝘆 𝗲𝗻𝗱𝗽𝗼𝗶𝗻𝘁𝘀. 𝗥𝗘𝗚𝗡-𝗖𝗢𝗩𝟮 𝘀𝗶𝗴𝗻𝗶𝗳𝗶𝗰𝗮𝗻𝘁𝗹𝘆 𝗿𝗲𝗱𝘂𝗰𝗲𝗱 𝘃𝗶𝗿𝗮𝗹 𝗹𝗼𝗮𝗱 𝗮𝗻𝗱 𝗽𝗮𝘁𝗶𝗲𝗻𝘁 𝗺𝗲𝗱𝗶𝗰𝗮𝗹 𝘃𝗶𝘀𝗶𝘁𝘀 (𝗵𝗼𝘀𝗽𝗶𝘁𝗮𝗹𝗶𝘇𝗮𝘁𝗶𝗼𝗻𝘀, 𝗲𝗺𝗲𝗿𝗴𝗲𝗻𝗰𝘆 𝗿𝗼𝗼𝗺, 𝘂𝗿𝗴𝗲𝗻𝘁 𝗰𝗮𝗿𝗲 𝘃𝗶𝘀𝗶𝘁𝘀 𝗮𝗻𝗱/𝗼𝗿 𝗽𝗵𝘆𝘀𝗶𝗰𝗶𝗮𝗻 𝗼𝗳𝗳𝗶𝗰𝗲/𝘁𝗲𝗹𝗲𝗺𝗲𝗱𝗶𝗰𝗶𝗻𝗲 𝘃𝗶𝘀𝗶𝘁𝘀).
"𝗧𝗵𝗲 𝗳𝗶𝗿𝘀𝘁 𝗷𝗼𝗯 𝗼𝗳 𝗮𝗻 𝗮𝗻𝘁𝗶𝘃𝗶𝗿𝗮𝗹 𝘁𝗵𝗲𝗿𝗮𝗽𝗲𝘂𝘁𝗶𝗰 𝗱𝗿𝘂𝗴 𝗶𝘀 𝘁𝗼 𝗹𝗼𝘄𝗲𝗿 𝘁𝗵𝗲 𝘃𝗶𝗿𝗮𝗹 𝗹𝗼𝗮𝗱, 𝗮𝗻𝗱 𝗼𝘂𝗿 𝗶𝗻𝗶𝘁𝗶𝗮𝗹 𝗱𝗮𝘁𝗮 𝗶𝗻 𝟮𝟳𝟱 𝗽𝗮𝘁𝗶𝗲𝗻𝘁𝘀 𝘀𝘁𝗿𝗼𝗻𝗴𝗹𝘆 𝘀𝘂𝗴𝗴𝗲𝘀𝘁𝗲𝗱 𝘁𝗵𝗮𝘁 𝘁𝗵𝗲 𝗥𝗘𝗚𝗡-𝗖𝗢𝗩𝟮 𝗮𝗻𝘁𝗶𝗯𝗼𝗱𝘆 𝗰𝗼𝗰𝗸𝘁𝗮𝗶𝗹 𝗰𝗼𝘂𝗹𝗱 𝗹𝗼𝘄𝗲𝗿 𝘃𝗶𝗿𝗮𝗹 𝗹𝗼𝗮𝗱 𝗮𝗻𝗱 𝘁𝗵𝗲𝗿𝗲𝗯𝘆 𝗽𝗼𝘁𝗲𝗻𝘁𝗶𝗮𝗹𝗹𝘆 𝗶𝗺𝗽𝗿𝗼𝘃𝗲 𝗰𝗹𝗶𝗻𝗶𝗰𝗮𝗹 𝗼𝘂𝘁𝗰𝗼𝗺𝗲𝘀. 𝗧𝗼𝗱𝗮𝘆'𝘀 𝗮𝗻𝗮𝗹𝘆𝘀𝗶𝘀, 𝗶𝗻𝘃𝗼𝗹𝘃𝗶𝗻𝗴 𝗺𝗼𝗿𝗲 𝘁𝗵𝗮𝗻 𝟱𝟬𝟬 𝗮𝗱𝗱𝗶𝘁𝗶𝗼𝗻𝗮𝗹 𝗽𝗮𝘁𝗶𝗲𝗻𝘁𝘀, 𝗽𝗿𝗼𝘀𝗽𝗲𝗰𝘁𝗶𝘃𝗲𝗹𝘆 𝗰𝗼𝗻𝗳𝗶𝗿𝗺𝘀 𝘁𝗵𝗮𝘁 𝗥𝗘𝗚𝗡-𝗖𝗢𝗩𝟮 𝗰𝗮𝗻 𝗶𝗻𝗱𝗲𝗲𝗱 𝘀𝗶𝗴𝗻𝗶𝗳𝗶𝗰𝗮𝗻𝘁𝗹𝘆 𝗿𝗲𝗱𝘂𝗰𝗲 𝘃𝗶𝗿𝗮𝗹 𝗹𝗼𝗮𝗱 𝗮𝗻𝗱 𝗳𝘂𝗿𝘁𝗵𝗲𝗿 𝘀𝗵𝗼𝘄𝘀 𝘁𝗵𝗮𝘁 𝘁𝗵𝗲𝘀𝗲 𝘃𝗶𝗿𝗮𝗹 𝗿𝗲𝗱𝘂𝗰𝘁𝗶𝗼𝗻𝘀 𝗮𝗿𝗲 𝗮𝘀𝘀𝗼𝗰𝗶𝗮𝘁𝗲𝗱 𝘄𝗶𝘁𝗵 𝗮 𝘀𝗶𝗴𝗻𝗶𝗳𝗶𝗰𝗮𝗻𝘁 𝗱𝗲𝗰𝗿𝗲𝗮𝘀𝗲 𝗶𝗻 𝘁𝗵𝗲 𝗻𝗲𝗲𝗱 𝗳𝗼𝗿 𝗳𝘂𝗿𝘁𝗵𝗲𝗿 𝗺𝗲𝗱𝗶𝗰𝗮𝗹 𝗮𝘁𝘁𝗲𝗻𝘁𝗶𝗼𝗻," 𝘀𝗮𝗶𝗱 𝗚𝗲𝗼𝗿𝗴𝗲 𝗗. 𝗬𝗮𝗻𝗰𝗼𝗽𝗼𝘂𝗹𝗼𝘀, 𝗠.𝗗., 𝗣𝗵.𝗗., 𝗣𝗿𝗲𝘀𝗶𝗱𝗲𝗻𝘁 𝗮𝗻𝗱 𝗖𝗵𝗶𝗲𝗳 𝗦𝗰𝗶𝗲𝗻𝘁𝗶𝗳𝗶𝗰 𝗢𝗳𝗳𝗶𝗰𝗲𝗿 𝗼𝗳 𝗥𝗲𝗴𝗲𝗻𝗲𝗿𝗼𝗻. "𝗪𝗲 𝗰𝗼𝗻𝘁𝗶𝗻𝘂𝗲 𝘁𝗼 𝘀𝗲𝗲 𝘁𝗵𝗲 𝘀𝘁𝗿𝗼𝗻𝗴𝗲𝘀𝘁 𝗲𝗳𝗳𝗲𝗰𝘁𝘀 𝗶𝗻 𝗽𝗮𝘁𝗶𝗲𝗻𝘁𝘀 𝘄𝗵𝗼 𝗮𝗿𝗲 𝗺𝗼𝘀𝘁 𝗮𝘁 𝗿𝗶𝘀𝗸 𝗳𝗼𝗿 𝗽𝗼𝗼𝗿 𝗼𝘂𝘁𝗰𝗼𝗺𝗲𝘀 𝗱𝘂𝗲 𝘁𝗼 𝗵𝗶𝗴𝗵 𝘃𝗶𝗿𝗮𝗹 𝗹𝗼𝗮𝗱, 𝗶𝗻𝗲𝗳𝗳𝗲𝗰𝘁𝗶𝘃𝗲 𝗮𝗻𝘁𝗶𝗯𝗼𝗱𝘆 𝗶𝗺𝗺𝘂𝗻𝗲 𝗿𝗲𝘀𝗽𝗼𝗻𝘀𝗲 𝗮𝘁 𝗯𝗮𝘀𝗲𝗹𝗶𝗻𝗲, 𝗼𝗿 𝗽𝗿𝗲-𝗲𝘅𝗶𝘀𝘁𝗶𝗻𝗴 𝗿𝗶𝘀𝗸 𝗳𝗮𝗰𝘁𝗼𝗿𝘀. 𝗥𝗲𝗴𝗲𝗻𝗲𝗿𝗼𝗻 𝗵𝗮𝘀 𝘀𝗵𝗮𝗿𝗲𝗱 𝘁𝗵𝗲𝘀𝗲 𝗿𝗲𝘀𝘂𝗹𝘁𝘀 𝘄𝗶𝘁𝗵 𝘁𝗵𝗲 𝗨.𝗦. 𝗙𝗼𝗼𝗱 𝗮𝗻𝗱 𝗗𝗿𝘂𝗴 𝗔𝗱𝗺𝗶𝗻𝗶𝘀𝘁𝗿𝗮𝘁𝗶𝗼𝗻 𝗮𝘀 𝗽𝗮𝗿𝘁 𝗼𝗳 𝗶𝘁𝘀 𝗿𝗲𝘃𝗶𝗲𝘄 𝗼𝗳 𝗼𝘂𝗿 𝗘𝗺𝗲𝗿𝗴𝗲𝗻𝗰𝘆 𝗨𝘀𝗲 𝗔𝘂𝘁𝗵𝗼𝗿𝗶𝘇𝗮𝘁𝗶𝗼𝗻 𝘀𝘂𝗯𝗺𝗶𝘀𝘀𝗶𝗼𝗻, 𝗮𝗻𝗱 𝘄𝗲 𝗰𝗼𝗻𝘁𝗶𝗻𝘂𝗲 𝘁𝗼 𝗳𝗼𝗰𝘂𝘀 𝗼𝗻 𝗰𝗼𝗺𝗽𝗹𝗲𝘁𝗶𝗻𝗴 𝗼𝘂𝗿 𝗼𝗻𝗴𝗼𝗶𝗻𝗴 𝘁𝗿𝗶𝗮𝗹𝘀 𝗲𝘃𝗮𝗹𝘂𝗮𝘁𝗶𝗻𝗴 𝗥𝗘𝗚𝗡-𝗖𝗢𝗩𝟮 𝗳𝗼𝗿 𝘁𝗵𝗲 𝘁𝗿𝗲𝗮𝘁𝗺𝗲𝗻𝘁 𝗮𝗻𝗱 𝗽𝗿𝗲𝘃𝗲𝗻𝘁𝗶𝗼𝗻 𝗼𝗳 𝗖𝗢𝗩𝗜𝗗-𝟭𝟵."
Certainly can't say it's a "cure" yet, but it's looking much better than any other drug out there, so far and could be a bridge to vaccines, especially as it's also being looked at as a prophylactic to prevent infection in high risk workers/possible patients. Regeneron has applied with the FDA for an Emergency Use Authorization soon, which I think makes sense. Yeah, I know - no COVID threads, but people ought to know about this. At least leave it up for a day or so. This is really important news given that Eli Lilly announced that they were stopping their trial in hospitalized COVID patients getting their single antibody treatment along with remdesivir, due to lack of efficacy (they also have a cocktail - awaiting results on that).
https://investor.regeneron.com/news...9-outpatient-trial-prospectively-demonstrates
𝗥𝗲𝗴𝗲𝗻𝗲𝗿𝗼𝗻 𝗣𝗵𝗮𝗿𝗺𝗮𝗰𝗲𝘂𝘁𝗶𝗰𝗮𝗹𝘀, 𝗜𝗻𝗰. (𝗡𝗔𝗦𝗗𝗔𝗤: 𝗥𝗘𝗚𝗡) 𝘁𝗼𝗱𝗮𝘆 𝗮𝗻𝗻𝗼𝘂𝗻𝗰𝗲𝗱 𝗽𝗼𝘀𝗶𝘁𝗶𝘃𝗲, 𝗽𝗿𝗼𝘀𝗽𝗲𝗰𝘁𝗶𝘃𝗲 𝗿𝗲𝘀𝘂𝗹𝘁𝘀 𝗳𝗿𝗼𝗺 𝗮𝗻 𝗼𝗻𝗴𝗼𝗶𝗻𝗴 𝗣𝗵𝗮𝘀𝗲 𝟮/𝟯 𝘀𝗲𝗮𝗺𝗹𝗲𝘀𝘀 𝘁𝗿𝗶𝗮𝗹 𝗶𝗻 𝘁𝗵𝗲 𝗖𝗢𝗩𝗜𝗗-𝟭𝟵 𝗼𝘂𝘁𝗽𝗮𝘁𝗶𝗲𝗻𝘁 𝘀𝗲𝘁𝘁𝗶𝗻𝗴 𝘀𝗵𝗼𝘄𝗶𝗻𝗴 𝗶𝘁𝘀 𝗶𝗻𝘃𝗲𝘀𝘁𝗶𝗴𝗮𝘁𝗶𝗼𝗻𝗮𝗹 𝗮𝗻𝘁𝗶𝗯𝗼𝗱𝘆 𝗰𝗼𝗰𝗸𝘁𝗮𝗶𝗹, 𝗥𝗘𝗚𝗡-𝗖𝗢𝗩𝟮, 𝗺𝗲𝘁 𝘁𝗵𝗲 𝗽𝗿𝗶𝗺𝗮𝗿𝘆 𝗮𝗻𝗱 𝗸𝗲𝘆 𝘀𝗲𝗰𝗼𝗻𝗱𝗮𝗿𝘆 𝗲𝗻𝗱𝗽𝗼𝗶𝗻𝘁𝘀. 𝗥𝗘𝗚𝗡-𝗖𝗢𝗩𝟮 𝘀𝗶𝗴𝗻𝗶𝗳𝗶𝗰𝗮𝗻𝘁𝗹𝘆 𝗿𝗲𝗱𝘂𝗰𝗲𝗱 𝘃𝗶𝗿𝗮𝗹 𝗹𝗼𝗮𝗱 𝗮𝗻𝗱 𝗽𝗮𝘁𝗶𝗲𝗻𝘁 𝗺𝗲𝗱𝗶𝗰𝗮𝗹 𝘃𝗶𝘀𝗶𝘁𝘀 (𝗵𝗼𝘀𝗽𝗶𝘁𝗮𝗹𝗶𝘇𝗮𝘁𝗶𝗼𝗻𝘀, 𝗲𝗺𝗲𝗿𝗴𝗲𝗻𝗰𝘆 𝗿𝗼𝗼𝗺, 𝘂𝗿𝗴𝗲𝗻𝘁 𝗰𝗮𝗿𝗲 𝘃𝗶𝘀𝗶𝘁𝘀 𝗮𝗻𝗱/𝗼𝗿 𝗽𝗵𝘆𝘀𝗶𝗰𝗶𝗮𝗻 𝗼𝗳𝗳𝗶𝗰𝗲/𝘁𝗲𝗹𝗲𝗺𝗲𝗱𝗶𝗰𝗶𝗻𝗲 𝘃𝗶𝘀𝗶𝘁𝘀).
"𝗧𝗵𝗲 𝗳𝗶𝗿𝘀𝘁 𝗷𝗼𝗯 𝗼𝗳 𝗮𝗻 𝗮𝗻𝘁𝗶𝘃𝗶𝗿𝗮𝗹 𝘁𝗵𝗲𝗿𝗮𝗽𝗲𝘂𝘁𝗶𝗰 𝗱𝗿𝘂𝗴 𝗶𝘀 𝘁𝗼 𝗹𝗼𝘄𝗲𝗿 𝘁𝗵𝗲 𝘃𝗶𝗿𝗮𝗹 𝗹𝗼𝗮𝗱, 𝗮𝗻𝗱 𝗼𝘂𝗿 𝗶𝗻𝗶𝘁𝗶𝗮𝗹 𝗱𝗮𝘁𝗮 𝗶𝗻 𝟮𝟳𝟱 𝗽𝗮𝘁𝗶𝗲𝗻𝘁𝘀 𝘀𝘁𝗿𝗼𝗻𝗴𝗹𝘆 𝘀𝘂𝗴𝗴𝗲𝘀𝘁𝗲𝗱 𝘁𝗵𝗮𝘁 𝘁𝗵𝗲 𝗥𝗘𝗚𝗡-𝗖𝗢𝗩𝟮 𝗮𝗻𝘁𝗶𝗯𝗼𝗱𝘆 𝗰𝗼𝗰𝗸𝘁𝗮𝗶𝗹 𝗰𝗼𝘂𝗹𝗱 𝗹𝗼𝘄𝗲𝗿 𝘃𝗶𝗿𝗮𝗹 𝗹𝗼𝗮𝗱 𝗮𝗻𝗱 𝘁𝗵𝗲𝗿𝗲𝗯𝘆 𝗽𝗼𝘁𝗲𝗻𝘁𝗶𝗮𝗹𝗹𝘆 𝗶𝗺𝗽𝗿𝗼𝘃𝗲 𝗰𝗹𝗶𝗻𝗶𝗰𝗮𝗹 𝗼𝘂𝘁𝗰𝗼𝗺𝗲𝘀. 𝗧𝗼𝗱𝗮𝘆'𝘀 𝗮𝗻𝗮𝗹𝘆𝘀𝗶𝘀, 𝗶𝗻𝘃𝗼𝗹𝘃𝗶𝗻𝗴 𝗺𝗼𝗿𝗲 𝘁𝗵𝗮𝗻 𝟱𝟬𝟬 𝗮𝗱𝗱𝗶𝘁𝗶𝗼𝗻𝗮𝗹 𝗽𝗮𝘁𝗶𝗲𝗻𝘁𝘀, 𝗽𝗿𝗼𝘀𝗽𝗲𝗰𝘁𝗶𝘃𝗲𝗹𝘆 𝗰𝗼𝗻𝗳𝗶𝗿𝗺𝘀 𝘁𝗵𝗮𝘁 𝗥𝗘𝗚𝗡-𝗖𝗢𝗩𝟮 𝗰𝗮𝗻 𝗶𝗻𝗱𝗲𝗲𝗱 𝘀𝗶𝗴𝗻𝗶𝗳𝗶𝗰𝗮𝗻𝘁𝗹𝘆 𝗿𝗲𝗱𝘂𝗰𝗲 𝘃𝗶𝗿𝗮𝗹 𝗹𝗼𝗮𝗱 𝗮𝗻𝗱 𝗳𝘂𝗿𝘁𝗵𝗲𝗿 𝘀𝗵𝗼𝘄𝘀 𝘁𝗵𝗮𝘁 𝘁𝗵𝗲𝘀𝗲 𝘃𝗶𝗿𝗮𝗹 𝗿𝗲𝗱𝘂𝗰𝘁𝗶𝗼𝗻𝘀 𝗮𝗿𝗲 𝗮𝘀𝘀𝗼𝗰𝗶𝗮𝘁𝗲𝗱 𝘄𝗶𝘁𝗵 𝗮 𝘀𝗶𝗴𝗻𝗶𝗳𝗶𝗰𝗮𝗻𝘁 𝗱𝗲𝗰𝗿𝗲𝗮𝘀𝗲 𝗶𝗻 𝘁𝗵𝗲 𝗻𝗲𝗲𝗱 𝗳𝗼𝗿 𝗳𝘂𝗿𝘁𝗵𝗲𝗿 𝗺𝗲𝗱𝗶𝗰𝗮𝗹 𝗮𝘁𝘁𝗲𝗻𝘁𝗶𝗼𝗻," 𝘀𝗮𝗶𝗱 𝗚𝗲𝗼𝗿𝗴𝗲 𝗗. 𝗬𝗮𝗻𝗰𝗼𝗽𝗼𝘂𝗹𝗼𝘀, 𝗠.𝗗., 𝗣𝗵.𝗗., 𝗣𝗿𝗲𝘀𝗶𝗱𝗲𝗻𝘁 𝗮𝗻𝗱 𝗖𝗵𝗶𝗲𝗳 𝗦𝗰𝗶𝗲𝗻𝘁𝗶𝗳𝗶𝗰 𝗢𝗳𝗳𝗶𝗰𝗲𝗿 𝗼𝗳 𝗥𝗲𝗴𝗲𝗻𝗲𝗿𝗼𝗻. "𝗪𝗲 𝗰𝗼𝗻𝘁𝗶𝗻𝘂𝗲 𝘁𝗼 𝘀𝗲𝗲 𝘁𝗵𝗲 𝘀𝘁𝗿𝗼𝗻𝗴𝗲𝘀𝘁 𝗲𝗳𝗳𝗲𝗰𝘁𝘀 𝗶𝗻 𝗽𝗮𝘁𝗶𝗲𝗻𝘁𝘀 𝘄𝗵𝗼 𝗮𝗿𝗲 𝗺𝗼𝘀𝘁 𝗮𝘁 𝗿𝗶𝘀𝗸 𝗳𝗼𝗿 𝗽𝗼𝗼𝗿 𝗼𝘂𝘁𝗰𝗼𝗺𝗲𝘀 𝗱𝘂𝗲 𝘁𝗼 𝗵𝗶𝗴𝗵 𝘃𝗶𝗿𝗮𝗹 𝗹𝗼𝗮𝗱, 𝗶𝗻𝗲𝗳𝗳𝗲𝗰𝘁𝗶𝘃𝗲 𝗮𝗻𝘁𝗶𝗯𝗼𝗱𝘆 𝗶𝗺𝗺𝘂𝗻𝗲 𝗿𝗲𝘀𝗽𝗼𝗻𝘀𝗲 𝗮𝘁 𝗯𝗮𝘀𝗲𝗹𝗶𝗻𝗲, 𝗼𝗿 𝗽𝗿𝗲-𝗲𝘅𝗶𝘀𝘁𝗶𝗻𝗴 𝗿𝗶𝘀𝗸 𝗳𝗮𝗰𝘁𝗼𝗿𝘀. 𝗥𝗲𝗴𝗲𝗻𝗲𝗿𝗼𝗻 𝗵𝗮𝘀 𝘀𝗵𝗮𝗿𝗲𝗱 𝘁𝗵𝗲𝘀𝗲 𝗿𝗲𝘀𝘂𝗹𝘁𝘀 𝘄𝗶𝘁𝗵 𝘁𝗵𝗲 𝗨.𝗦. 𝗙𝗼𝗼𝗱 𝗮𝗻𝗱 𝗗𝗿𝘂𝗴 𝗔𝗱𝗺𝗶𝗻𝗶𝘀𝘁𝗿𝗮𝘁𝗶𝗼𝗻 𝗮𝘀 𝗽𝗮𝗿𝘁 𝗼𝗳 𝗶𝘁𝘀 𝗿𝗲𝘃𝗶𝗲𝘄 𝗼𝗳 𝗼𝘂𝗿 𝗘𝗺𝗲𝗿𝗴𝗲𝗻𝗰𝘆 𝗨𝘀𝗲 𝗔𝘂𝘁𝗵𝗼𝗿𝗶𝘇𝗮𝘁𝗶𝗼𝗻 𝘀𝘂𝗯𝗺𝗶𝘀𝘀𝗶𝗼𝗻, 𝗮𝗻𝗱 𝘄𝗲 𝗰𝗼𝗻𝘁𝗶𝗻𝘂𝗲 𝘁𝗼 𝗳𝗼𝗰𝘂𝘀 𝗼𝗻 𝗰𝗼𝗺𝗽𝗹𝗲𝘁𝗶𝗻𝗴 𝗼𝘂𝗿 𝗼𝗻𝗴𝗼𝗶𝗻𝗴 𝘁𝗿𝗶𝗮𝗹𝘀 𝗲𝘃𝗮𝗹𝘂𝗮𝘁𝗶𝗻𝗴 𝗥𝗘𝗚𝗡-𝗖𝗢𝗩𝟮 𝗳𝗼𝗿 𝘁𝗵𝗲 𝘁𝗿𝗲𝗮𝘁𝗺𝗲𝗻𝘁 𝗮𝗻𝗱 𝗽𝗿𝗲𝘃𝗲𝗻𝘁𝗶𝗼𝗻 𝗼𝗳 𝗖𝗢𝗩𝗜𝗗-𝟭𝟵."