This might not be everyone's cup of tea, but thought it was a very cool research article on innovation in synthetic organic chemistry used to discover "new molecular entity" small molecule drugs (i.e., drugs that are small enough in molecular weight to be 100% characterized chemically; most monoclonal antibodies, proteins, vaccines, etc. cannot, so were not part of this study) that went on to be approved by the FDA for treatment of some disease. The analysis looked at 1089 such molecules approved since 1938 and shows that pharmaceutical innovation (from a chemical structure perspective) has increased considerably over the past 20 years, which is good to see, as that has been questioned.
https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00319#
The authors, from the Chemical Abstracts Registry, grouped these molecules into three main categories of innovation: "pioneers," which are molecules with uniquely new shapes and scaffolds, "settlers," which are molecules with uniquely new scaffolds, but not novel shapes, and "colonists," which are molecules with neither new shapes or scaffolds (i.e., the least innovative molecules).
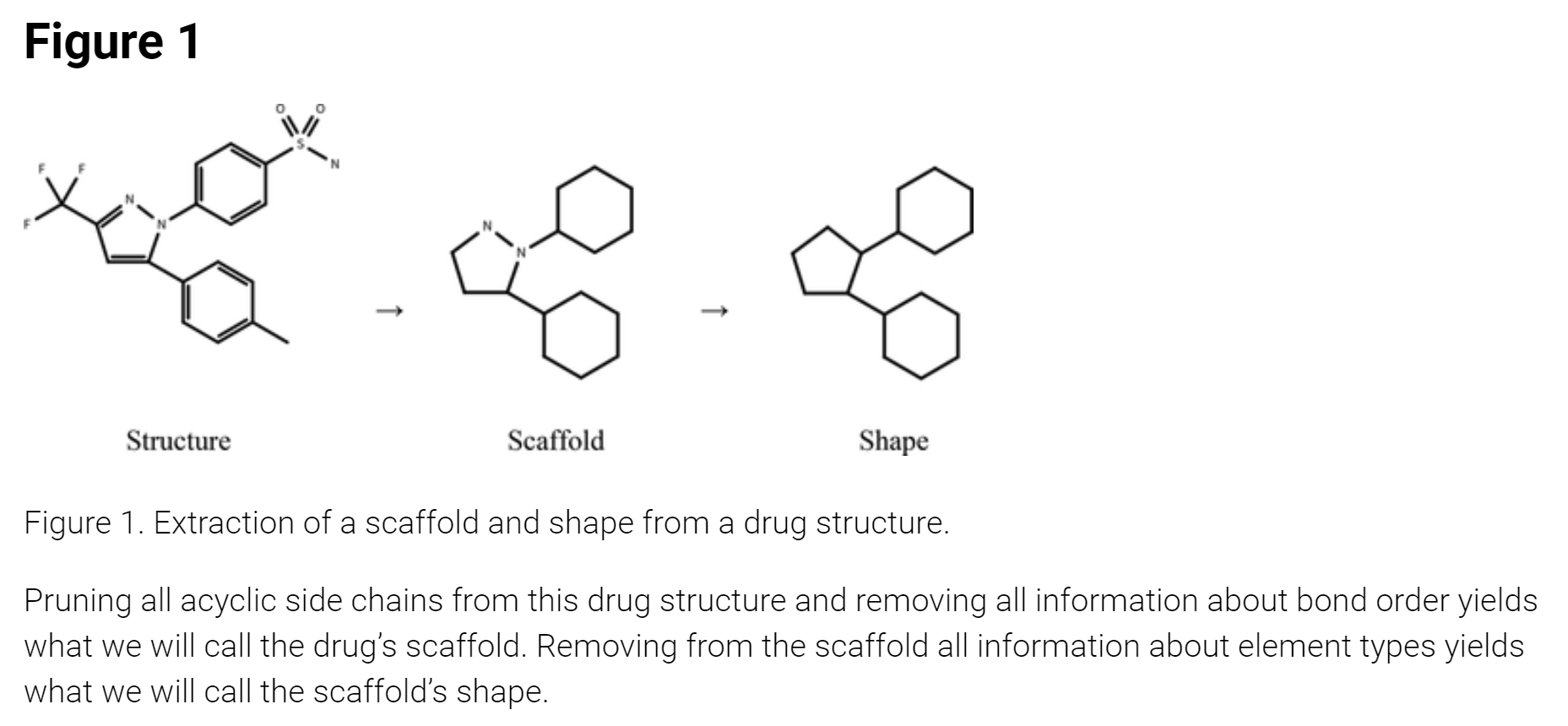
The graphic below illustrates how one extracts the "scaffold" from the actual drug structure, by removing the acyclic side-chains from the structure (just focusing on the cyclic ring structure), and how one extracts the "shape" by removing the elemental bond information from the scaffold (i.e., assuming all elements are carbon). The example is of CELEBREX, the COX-2 inhibitor for pain relief.
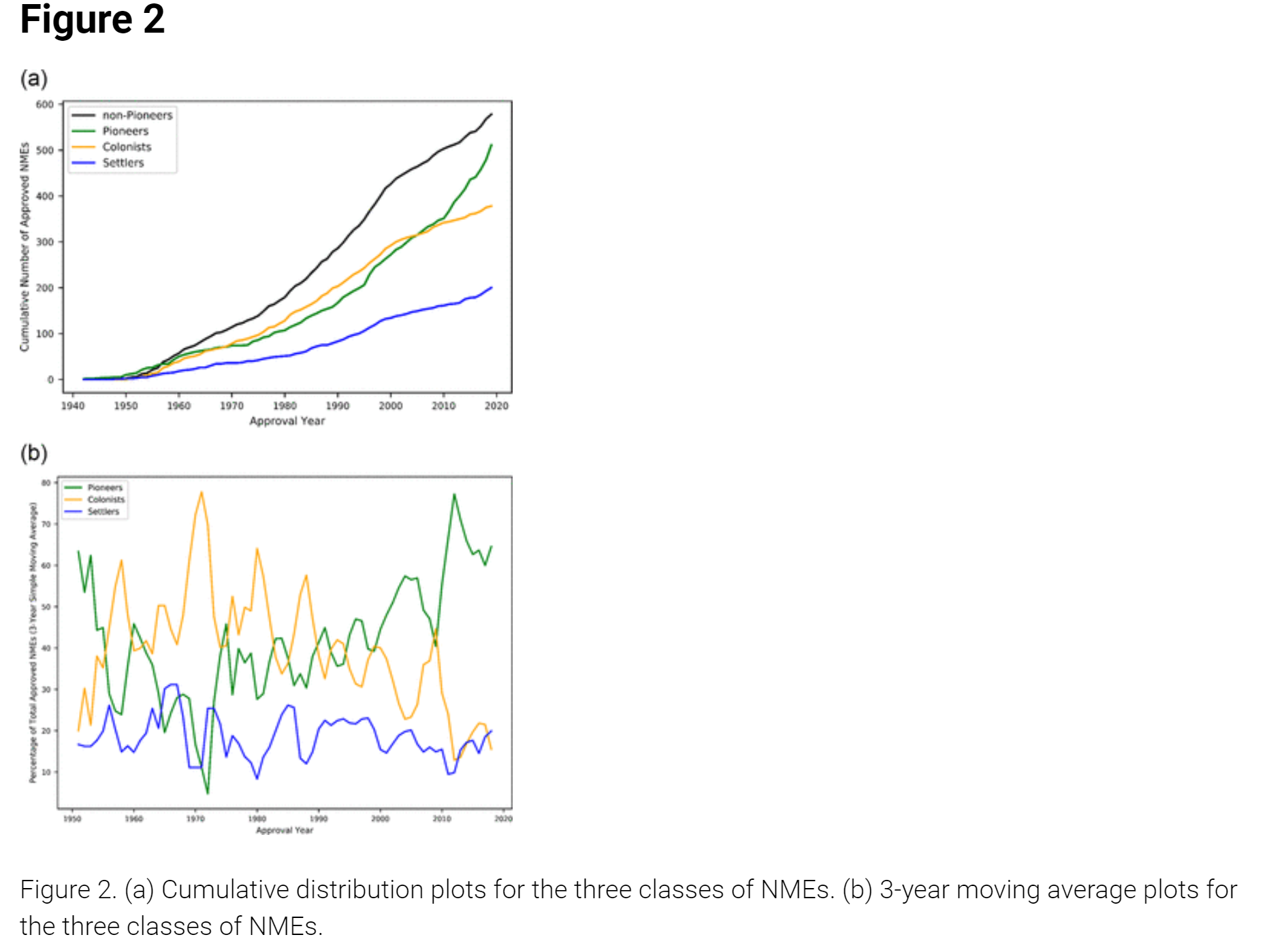
As is often the case, Derek Lowe covered this article nicely in his In the Pipeline blog, as soon as it came out, which is not surprising, as he is a synthetic organic medicinal chemist by training and has spent his whole career trying to discover new chemical matter of pharmaceutical relevance, so this was right up his alley. He summarizes the results from the ACS paper very nicely (and much more concisely than the authors), below, highlighting how innovation has been on the increase in the last 20 years as depicted in the 2nd figure, below, showing the increase in "pioneer" molecules since about 2000.
I've worked with chemists like the authors and Derek my whole career and have always been in awe of their ability to "see" or "invent" a molecule - and/or figure out its synthesis largely in their heads - as a chemical engineer, I'm conversant with organic chemistry, but I don't "live it" like these guys do - I used to joke that they're all just stick figures to me.
https://blogs.sciencemag.org/.../new-drugs-and-new...
𝗜𝘁 𝘁𝘂𝗿𝗻𝘀 𝗼𝘂𝘁 𝘁𝗵𝗮𝘁 𝗣𝗶𝗼𝗻𝗲𝗲𝗿 𝗺𝗼𝗹𝗲𝗰𝘂𝗹𝗲𝘀 𝗮𝗿𝗲 𝗶𝗻 𝗳𝗮𝗰𝘁 𝗼𝘃𝗲𝗿-𝗿𝗲𝗽𝗿𝗲𝘀𝗲𝗻𝘁𝗲𝗱 𝗶𝗻 𝗹𝗶𝘀𝘁𝘀 𝗼𝗳 𝘁𝗵𝗲𝗿𝗮𝗽𝗲𝘂𝘁𝗶𝗰𝗮𝗹𝗹𝘆 𝗶𝗻𝗻𝗼𝘃𝗮𝘁𝗶𝘃𝗲 𝗺𝗼𝗹𝗲𝗰𝘂𝗹𝗲𝘀, 𝘄𝗵𝗶𝗰𝗵 𝗶𝘀 𝗴𝗼𝗼𝗱 𝘁𝗼 𝗸𝗻𝗼𝘄 𝗳𝗼𝗿 𝘀𝘂𝗿𝗲. 𝗔𝘀 𝗮 𝘀𝘆𝗻𝘁𝗵𝗲𝘁𝗶𝗰 𝗰𝗵𝗲𝗺𝗶𝘀𝘁, 𝘁𝗵𝗮𝘁’𝘀 𝘄𝗵𝗮𝘁 𝘆𝗼𝘂 𝘄𝗼𝘂𝗹𝗱 𝗹𝗶𝗸𝗲 𝘁𝗼 𝗵𝗲𝗮𝗿, 𝗯𝘂𝘁 𝗶𝘁’𝘀 𝗴𝗼𝗼𝗱 𝘁𝗼 𝘀𝗲𝗲 𝗶𝘁 𝗾𝘂𝗮𝗻𝘁𝗶𝗳𝗶𝗲𝗱. 𝗔𝗻𝗱 𝗣𝗶𝗼𝗻𝗲𝗲𝗿𝘀 𝗮𝗿𝗲 𝗳𝗼𝗹𝗹𝗼𝘄𝗲𝗱 (𝗲𝘃𝗲𝗻𝘁𝘂𝗮𝗹𝗹𝘆) 𝗮𝗿𝗼𝘂𝗻𝗱 𝟮𝟬% 𝗼𝗳 𝘁𝗵𝗲 𝘁𝗶𝗺𝗲 𝗯𝘆 𝗿𝗼𝘂𝗴𝗵𝗹𝘆 𝘀𝗶𝗺𝗶𝗹𝗮𝗿 𝗦𝗲𝘁𝘁𝗹𝗲𝗿 𝗺𝗼𝗹𝗲𝗰𝘂𝗹𝗲𝘀, 𝗮𝗻𝗱 𝗦𝗲𝘁𝘁𝗹𝗲𝗿 𝗺𝗼𝗹𝗲𝗰𝘂𝗹𝗲𝘀 𝗮𝗿𝗲 𝗲𝘃𝗲𝗻𝘁𝘂𝗮𝗹𝗹𝘆 𝗳𝗼𝗹𝗹𝗼𝘄𝗲𝗱 𝗮𝗯𝗼𝘂𝘁 𝟮𝟬% 𝗼𝗳 𝘁𝗵𝗲 𝘁𝗶𝗺𝗲 𝗯𝘆 𝘀𝗶𝗺𝗶𝗹𝗮𝗿 𝗖𝗼𝗹𝗼𝗻𝗶𝘀𝘁 𝗼𝗻𝗲𝘀. 𝗦𝘁𝘂𝗱𝗲𝗻𝘁𝘀 𝗼𝗳 𝗺𝗲𝗱-𝗰𝗵𝗲𝗺 𝗵𝗶𝘀𝘁𝗼𝗿𝘆 𝗺𝗮𝘆 𝗯𝗲 𝗿𝗲𝗺𝗶𝗻𝗱𝗲𝗱 𝗼𝗳 𝗦𝗶𝗿 𝗝𝗮𝗺𝗲𝘀 𝗕𝗹𝗮𝗰𝗸’𝘀 𝗹𝗶𝗻𝗲 𝗮𝗯𝗼𝘂𝘁 𝗵𝗼𝘄 𝘁𝗵𝗲 𝗯𝗲𝘀𝘁 𝘄𝗮𝘆 𝘁𝗼 𝗳𝗶𝗻𝗱 𝗮 𝗻𝗲𝘄 𝗱𝗿𝘂𝗴 𝗶𝘀 𝘁𝗼 𝘀𝘁𝗮𝗿𝘁 𝘄𝗶𝘁𝗵 𝗮𝗻 𝗼𝗹𝗱 𝗱𝗿𝘂𝗴! 𝗔𝗻𝗱 𝗶𝗻𝗱𝗲𝗲𝗱, 𝗖𝗼𝗹𝗼𝗻𝗶𝘀𝘁-𝗰𝗹𝗮𝘀𝘀 𝗱𝗿𝘂𝗴 𝘀𝘁𝗿𝘂𝗰𝘁𝘂𝗿𝗲𝘀 𝘄𝗲𝗿𝗲 𝘁𝗵𝗲 𝗺𝗼𝘀𝘁 𝗻𝘂𝗺𝗲𝗿𝗼𝘂𝘀 𝗼𝗳 𝘁𝗵𝗲 𝘁𝗵𝗿𝗲𝗲 𝗶𝗻 𝗙𝗗𝗔 𝗮𝗽𝗽𝗿𝗼𝘃𝗮𝗹𝘀 𝗳𝗿𝗼𝗺 𝗮𝗯𝗼𝘂𝘁 𝟭𝟵𝟳𝟬 𝘁𝗼 𝘁𝗵𝗲 𝗲𝗮𝗿𝗹𝘆 𝟮𝟬𝟬𝟬𝘀, 𝗯𝘂𝘁 𝘀𝗶𝗻𝗰𝗲 𝘁𝗵𝗲𝗻, 𝗣𝗶𝗼𝗻𝗲𝗲𝗿 𝘁𝘆𝗽𝗲 𝘀𝘁𝗿𝘂𝗰𝘁𝘂𝗿𝗲𝘀 𝗵𝗮𝘃𝗲 𝘁𝗮𝗸𝗲𝗻 𝗼𝗳𝗳 𝗮𝗻𝗱 𝗲𝘀𝘁𝗮𝗯𝗹𝗶𝘀𝗵𝗲𝗱 𝗮𝗻 𝘂𝗻𝗽𝗿𝗲𝗰𝗲𝗱𝗲𝗻𝘁𝗲𝗱 𝗹𝗲𝗮𝗱. 𝗜𝘁 𝗮𝗽𝗽𝗲𝗮𝗿𝘀 𝘁𝗵𝗮𝘁 𝘁𝗵𝗶𝘀 𝗶𝘀 𝗱𝘂𝗲 𝘁𝗼 𝘁𝘄𝗼 𝘀𝗶𝗺𝘂𝗹𝘁𝗮𝗻𝗲𝗼𝘂𝘀 𝗳𝗮𝗰𝘁𝗼𝗿𝘀 – 𝘁𝗵𝗲 𝗻𝘂𝗺𝗯𝗲𝗿 𝗼𝗳 𝗣𝗶𝗼𝗻𝗲𝗲𝗿 𝘀𝘁𝗿𝘂𝗰𝘁𝘂𝗿𝗲𝘀 𝗵𝗮𝘀 𝗶𝗻𝗰𝗿𝗲𝗮𝘀𝗲𝗱 (𝗮𝗻𝗱 𝗵𝗮𝘀 𝗯𝗲𝗲𝗻 𝗶𝗻𝗰𝗿𝗲𝗮𝘀𝗶𝗻𝗴, 𝗶𝗻 𝗮 𝗿𝗼𝘂𝗴𝗵 𝗮𝗻𝗱 𝗻𝗼𝘄 𝗮𝗰𝗰𝗲𝗹𝗲𝗿𝗮𝘁𝗶𝗻𝗴 𝘄𝗮𝘆, 𝗲𝘃𝗲𝗿 𝘀𝗶𝗻𝗰𝗲 𝘀𝗼𝗺𝗲𝘁𝗶𝗺𝗲 𝗶𝗻 𝘁𝗵𝗲 𝟭𝟵𝟵𝟬𝘀), 𝘄𝗵𝗶𝗹𝗲 𝘁𝗵𝗲 𝗻𝘂𝗺𝗯𝗲𝗿 𝗼𝗳 𝗖𝗼𝗹𝗼𝗻𝗶𝘀𝘁 𝘀𝘁𝗿𝘂𝗰𝘁𝘂𝗿𝗲𝘀 𝗵𝗮𝘀 𝗯𝗲𝗲𝗻 𝗱𝗿𝗼𝗽𝗽𝗶𝗻𝗴 𝗻𝗼𝘁𝗶𝗰𝗲𝗮𝗯𝗹𝘆. (𝗧𝗵𝗲 𝗶𝗻𝘁𝗲𝗿𝗺𝗲𝗱𝗶𝗮𝘁𝗲 𝗰𝗮𝘁𝗲𝗴𝗼𝗿𝘆 𝗼𝗳 𝗦𝗲𝘁𝘁𝗹𝗲𝗿 𝗰𝗼𝗺𝗽𝗼𝘂𝗻𝗱𝘀 𝗵𝗮𝘀 𝗯𝗲𝗲𝗻 𝗯𝗮𝗻𝗴𝗶𝗻𝗴 𝗮𝗹𝗼𝗻𝗴 𝗶𝗻 𝘁𝗵𝗶𝗿𝗱 𝗽𝗹𝗮𝗰𝗲 𝗮𝘁 𝗿𝗼𝘂𝗴𝗵𝗹𝘆 𝘀𝗶𝗺𝗶𝗹𝗮𝗿 𝗹𝗲𝘃𝗲𝗹𝘀 𝘁𝗵𝗲 𝘄𝗵𝗼𝗹𝗲 𝘁𝗶𝗺𝗲, 𝗶𝗻 𝗰𝗮𝘀𝗲 𝘆𝗼𝘂 𝘄𝗲𝗿𝗲 𝘄𝗼𝗻𝗱𝗲𝗿𝗶𝗻𝗴).
https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00319#
The authors, from the Chemical Abstracts Registry, grouped these molecules into three main categories of innovation: "pioneers," which are molecules with uniquely new shapes and scaffolds, "settlers," which are molecules with uniquely new scaffolds, but not novel shapes, and "colonists," which are molecules with neither new shapes or scaffolds (i.e., the least innovative molecules).
The graphic below illustrates how one extracts the "scaffold" from the actual drug structure, by removing the acyclic side-chains from the structure (just focusing on the cyclic ring structure), and how one extracts the "shape" by removing the elemental bond information from the scaffold (i.e., assuming all elements are carbon). The example is of CELEBREX, the COX-2 inhibitor for pain relief.
As is often the case, Derek Lowe covered this article nicely in his In the Pipeline blog, as soon as it came out, which is not surprising, as he is a synthetic organic medicinal chemist by training and has spent his whole career trying to discover new chemical matter of pharmaceutical relevance, so this was right up his alley. He summarizes the results from the ACS paper very nicely (and much more concisely than the authors), below, highlighting how innovation has been on the increase in the last 20 years as depicted in the 2nd figure, below, showing the increase in "pioneer" molecules since about 2000.
I've worked with chemists like the authors and Derek my whole career and have always been in awe of their ability to "see" or "invent" a molecule - and/or figure out its synthesis largely in their heads - as a chemical engineer, I'm conversant with organic chemistry, but I don't "live it" like these guys do - I used to joke that they're all just stick figures to me.
https://blogs.sciencemag.org/.../new-drugs-and-new...
𝗜𝘁 𝘁𝘂𝗿𝗻𝘀 𝗼𝘂𝘁 𝘁𝗵𝗮𝘁 𝗣𝗶𝗼𝗻𝗲𝗲𝗿 𝗺𝗼𝗹𝗲𝗰𝘂𝗹𝗲𝘀 𝗮𝗿𝗲 𝗶𝗻 𝗳𝗮𝗰𝘁 𝗼𝘃𝗲𝗿-𝗿𝗲𝗽𝗿𝗲𝘀𝗲𝗻𝘁𝗲𝗱 𝗶𝗻 𝗹𝗶𝘀𝘁𝘀 𝗼𝗳 𝘁𝗵𝗲𝗿𝗮𝗽𝗲𝘂𝘁𝗶𝗰𝗮𝗹𝗹𝘆 𝗶𝗻𝗻𝗼𝘃𝗮𝘁𝗶𝘃𝗲 𝗺𝗼𝗹𝗲𝗰𝘂𝗹𝗲𝘀, 𝘄𝗵𝗶𝗰𝗵 𝗶𝘀 𝗴𝗼𝗼𝗱 𝘁𝗼 𝗸𝗻𝗼𝘄 𝗳𝗼𝗿 𝘀𝘂𝗿𝗲. 𝗔𝘀 𝗮 𝘀𝘆𝗻𝘁𝗵𝗲𝘁𝗶𝗰 𝗰𝗵𝗲𝗺𝗶𝘀𝘁, 𝘁𝗵𝗮𝘁’𝘀 𝘄𝗵𝗮𝘁 𝘆𝗼𝘂 𝘄𝗼𝘂𝗹𝗱 𝗹𝗶𝗸𝗲 𝘁𝗼 𝗵𝗲𝗮𝗿, 𝗯𝘂𝘁 𝗶𝘁’𝘀 𝗴𝗼𝗼𝗱 𝘁𝗼 𝘀𝗲𝗲 𝗶𝘁 𝗾𝘂𝗮𝗻𝘁𝗶𝗳𝗶𝗲𝗱. 𝗔𝗻𝗱 𝗣𝗶𝗼𝗻𝗲𝗲𝗿𝘀 𝗮𝗿𝗲 𝗳𝗼𝗹𝗹𝗼𝘄𝗲𝗱 (𝗲𝘃𝗲𝗻𝘁𝘂𝗮𝗹𝗹𝘆) 𝗮𝗿𝗼𝘂𝗻𝗱 𝟮𝟬% 𝗼𝗳 𝘁𝗵𝗲 𝘁𝗶𝗺𝗲 𝗯𝘆 𝗿𝗼𝘂𝗴𝗵𝗹𝘆 𝘀𝗶𝗺𝗶𝗹𝗮𝗿 𝗦𝗲𝘁𝘁𝗹𝗲𝗿 𝗺𝗼𝗹𝗲𝗰𝘂𝗹𝗲𝘀, 𝗮𝗻𝗱 𝗦𝗲𝘁𝘁𝗹𝗲𝗿 𝗺𝗼𝗹𝗲𝗰𝘂𝗹𝗲𝘀 𝗮𝗿𝗲 𝗲𝘃𝗲𝗻𝘁𝘂𝗮𝗹𝗹𝘆 𝗳𝗼𝗹𝗹𝗼𝘄𝗲𝗱 𝗮𝗯𝗼𝘂𝘁 𝟮𝟬% 𝗼𝗳 𝘁𝗵𝗲 𝘁𝗶𝗺𝗲 𝗯𝘆 𝘀𝗶𝗺𝗶𝗹𝗮𝗿 𝗖𝗼𝗹𝗼𝗻𝗶𝘀𝘁 𝗼𝗻𝗲𝘀. 𝗦𝘁𝘂𝗱𝗲𝗻𝘁𝘀 𝗼𝗳 𝗺𝗲𝗱-𝗰𝗵𝗲𝗺 𝗵𝗶𝘀𝘁𝗼𝗿𝘆 𝗺𝗮𝘆 𝗯𝗲 𝗿𝗲𝗺𝗶𝗻𝗱𝗲𝗱 𝗼𝗳 𝗦𝗶𝗿 𝗝𝗮𝗺𝗲𝘀 𝗕𝗹𝗮𝗰𝗸’𝘀 𝗹𝗶𝗻𝗲 𝗮𝗯𝗼𝘂𝘁 𝗵𝗼𝘄 𝘁𝗵𝗲 𝗯𝗲𝘀𝘁 𝘄𝗮𝘆 𝘁𝗼 𝗳𝗶𝗻𝗱 𝗮 𝗻𝗲𝘄 𝗱𝗿𝘂𝗴 𝗶𝘀 𝘁𝗼 𝘀𝘁𝗮𝗿𝘁 𝘄𝗶𝘁𝗵 𝗮𝗻 𝗼𝗹𝗱 𝗱𝗿𝘂𝗴! 𝗔𝗻𝗱 𝗶𝗻𝗱𝗲𝗲𝗱, 𝗖𝗼𝗹𝗼𝗻𝗶𝘀𝘁-𝗰𝗹𝗮𝘀𝘀 𝗱𝗿𝘂𝗴 𝘀𝘁𝗿𝘂𝗰𝘁𝘂𝗿𝗲𝘀 𝘄𝗲𝗿𝗲 𝘁𝗵𝗲 𝗺𝗼𝘀𝘁 𝗻𝘂𝗺𝗲𝗿𝗼𝘂𝘀 𝗼𝗳 𝘁𝗵𝗲 𝘁𝗵𝗿𝗲𝗲 𝗶𝗻 𝗙𝗗𝗔 𝗮𝗽𝗽𝗿𝗼𝘃𝗮𝗹𝘀 𝗳𝗿𝗼𝗺 𝗮𝗯𝗼𝘂𝘁 𝟭𝟵𝟳𝟬 𝘁𝗼 𝘁𝗵𝗲 𝗲𝗮𝗿𝗹𝘆 𝟮𝟬𝟬𝟬𝘀, 𝗯𝘂𝘁 𝘀𝗶𝗻𝗰𝗲 𝘁𝗵𝗲𝗻, 𝗣𝗶𝗼𝗻𝗲𝗲𝗿 𝘁𝘆𝗽𝗲 𝘀𝘁𝗿𝘂𝗰𝘁𝘂𝗿𝗲𝘀 𝗵𝗮𝘃𝗲 𝘁𝗮𝗸𝗲𝗻 𝗼𝗳𝗳 𝗮𝗻𝗱 𝗲𝘀𝘁𝗮𝗯𝗹𝗶𝘀𝗵𝗲𝗱 𝗮𝗻 𝘂𝗻𝗽𝗿𝗲𝗰𝗲𝗱𝗲𝗻𝘁𝗲𝗱 𝗹𝗲𝗮𝗱. 𝗜𝘁 𝗮𝗽𝗽𝗲𝗮𝗿𝘀 𝘁𝗵𝗮𝘁 𝘁𝗵𝗶𝘀 𝗶𝘀 𝗱𝘂𝗲 𝘁𝗼 𝘁𝘄𝗼 𝘀𝗶𝗺𝘂𝗹𝘁𝗮𝗻𝗲𝗼𝘂𝘀 𝗳𝗮𝗰𝘁𝗼𝗿𝘀 – 𝘁𝗵𝗲 𝗻𝘂𝗺𝗯𝗲𝗿 𝗼𝗳 𝗣𝗶𝗼𝗻𝗲𝗲𝗿 𝘀𝘁𝗿𝘂𝗰𝘁𝘂𝗿𝗲𝘀 𝗵𝗮𝘀 𝗶𝗻𝗰𝗿𝗲𝗮𝘀𝗲𝗱 (𝗮𝗻𝗱 𝗵𝗮𝘀 𝗯𝗲𝗲𝗻 𝗶𝗻𝗰𝗿𝗲𝗮𝘀𝗶𝗻𝗴, 𝗶𝗻 𝗮 𝗿𝗼𝘂𝗴𝗵 𝗮𝗻𝗱 𝗻𝗼𝘄 𝗮𝗰𝗰𝗲𝗹𝗲𝗿𝗮𝘁𝗶𝗻𝗴 𝘄𝗮𝘆, 𝗲𝘃𝗲𝗿 𝘀𝗶𝗻𝗰𝗲 𝘀𝗼𝗺𝗲𝘁𝗶𝗺𝗲 𝗶𝗻 𝘁𝗵𝗲 𝟭𝟵𝟵𝟬𝘀), 𝘄𝗵𝗶𝗹𝗲 𝘁𝗵𝗲 𝗻𝘂𝗺𝗯𝗲𝗿 𝗼𝗳 𝗖𝗼𝗹𝗼𝗻𝗶𝘀𝘁 𝘀𝘁𝗿𝘂𝗰𝘁𝘂𝗿𝗲𝘀 𝗵𝗮𝘀 𝗯𝗲𝗲𝗻 𝗱𝗿𝗼𝗽𝗽𝗶𝗻𝗴 𝗻𝗼𝘁𝗶𝗰𝗲𝗮𝗯𝗹𝘆. (𝗧𝗵𝗲 𝗶𝗻𝘁𝗲𝗿𝗺𝗲𝗱𝗶𝗮𝘁𝗲 𝗰𝗮𝘁𝗲𝗴𝗼𝗿𝘆 𝗼𝗳 𝗦𝗲𝘁𝘁𝗹𝗲𝗿 𝗰𝗼𝗺𝗽𝗼𝘂𝗻𝗱𝘀 𝗵𝗮𝘀 𝗯𝗲𝗲𝗻 𝗯𝗮𝗻𝗴𝗶𝗻𝗴 𝗮𝗹𝗼𝗻𝗴 𝗶𝗻 𝘁𝗵𝗶𝗿𝗱 𝗽𝗹𝗮𝗰𝗲 𝗮𝘁 𝗿𝗼𝘂𝗴𝗵𝗹𝘆 𝘀𝗶𝗺𝗶𝗹𝗮𝗿 𝗹𝗲𝘃𝗲𝗹𝘀 𝘁𝗵𝗲 𝘄𝗵𝗼𝗹𝗲 𝘁𝗶𝗺𝗲, 𝗶𝗻 𝗰𝗮𝘀𝗲 𝘆𝗼𝘂 𝘄𝗲𝗿𝗲 𝘄𝗼𝗻𝗱𝗲𝗿𝗶𝗻𝗴).







