Much more data in today in a paper by Joyner et al (preprint), evaluating the use of convalescent plasma in 5000 severely ill COVID-19 patients participating in the large observational trial (no control arm - essentially an emergency use "expanded access program") being run by the Mayo Clinic. Bottom line is it appears to be safe and "promising" with respect to lowering morality rates, although the study wasn't designed to evaluate that.
https://www.medrxiv.org/content/10.1101/2020.05.12.20099879v1.full.pdf
First off they concluded that CP is generally "safe" in the context of not adding additional risk to patients - all 5000 patients had "severe or life-threatening" cases of COVID-19. With regard to effectiveness, the analysis wasn't designed to evaluate that specifically, but they shared some data on 7-day mortality and to me the most striking data was that of the 3,316 patients admitted to the ICU, 456 mortalities were observed (16.7% after adjustments) vs. the typical mortality rate for ICU patients of 57% - that's a 71% reduction in morality rate for patients in the ICU and this wasn't a 10-person study or even a 46-person study (with 60% mortality reduction with CP), like the one above. However, it was only a 7-day analysis and perhaps those mortality numbers will increase significantly over time - would be nice to know what percentage of deaths occur beyond 7 days for such severely ill patients.
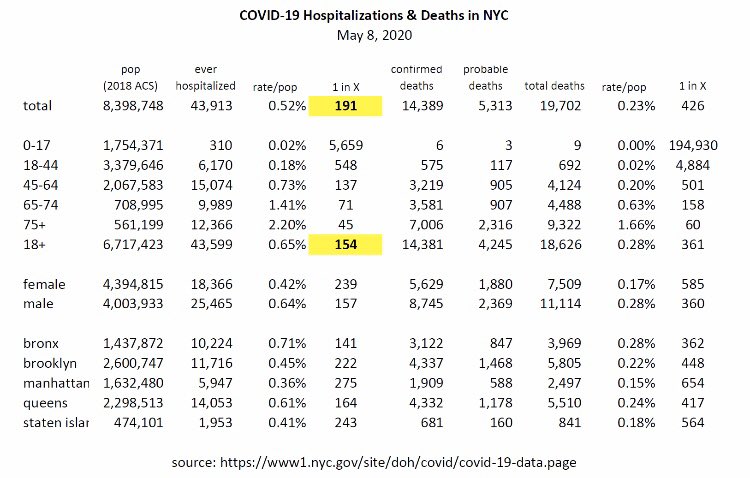
Of the 1682 hospitalized patients not admitted to the ICU the 7-day mortality rate was 11.2% vs. 15-20% typical for hospitalized patients, more like a 25-45% reduction; again, this was a 7-day mortality rate. However, NYC hospitalization mortality rates are about 33%, so the overall hospitalization mortality rate for this study, so far at 7 days (so not complete data yet) of 14.9% would be a 55% reduction. As an aside, I was a bit confused here, though - I would have thought patients sick enough to eventually die would go into the ICU first.
Anyway, it's not time to go out and celebrate yet, as this was not a placebo (plasma without antibodies)/standard of care controlled randomized double blind clinical study (that is ongoing), meaning the study was not designed to evaluate efficacy of CP per se, as per the excerpt below. However, the data above, especially the ICU data, are still pretty impressive, IMO, although it's only 7-day mortality data, so it's not complete yet. It's also a little bit odd that they didn't include the 57% typical ICU mortality rate comment together with the 13.7% ICU mortality rate data in this study - it's almost as if they don't want people hyping the potentially positive result (which I'm trying not to do; just sharing the data).
Over the first seven days after the convalescent plasma transfusion, a total of 602 mortalities were observed. The overall seven-day mortality rate was estimated to be 14.9% (95% CI: 13.8%, 16.0%) using the product limit estimator; an estimate that was numerically higher than the crude estimate of 12.0% at day 7. Of the 3,316 patients admitted to the ICU, 456 mortalities were observed (16.7%, 95% CI: 15.3%, 18.1%). Of the 1,682 hospitalized patients not-admitted to the ICU, 146 mortalities were observed (11.2%, 95% CI: 9.5%, 12.9%)...
...Although this study was not designed to evaluate efficacy of convalescent plasma we note with optimism the relatively low mortality in treated patients. The case fatality rate of COVID-19 has been reported to be ~4% among all persons diagnosed with COVID-19 (2); however, the case fatality rate among hospitalized patients is much higher ~15-20% (3, 5) and even more so among patients admitted to the ICU (57%) (4). Thus, the seven-day mortality rate was 14.9% reported here is not alarming, particularly because some of these plasma transfusions may be characterized as attempts at rescue or salvage therapy in patients admitted to the ICU with multi-organ failure, sepsis and significant comorbidities.
In addition, it's also noteworthy that the study did not find any obvious evidence of "antibody dependent enhancement" (ADE), which is a theoretical concern of the use of convalescent plasma, which could lead to "deteriorated clinical condition after plasma transfusion secondary to antibody-dependent enhancement (ADE) of infection or antibody-mediated proinflammatory effects." Limited evidence of this was seen with antibody therapies for other coronaviruses.
The absence of a toxicity signature with the use of convalescent plasma in individuals with COVID-19 implies that this phenomenon may be clinically inconsequential. COVID-19 is known to elicit high neutralizing antibody titers in individuals who have recently recovered from infection and three case series of convalescent plasma administration also describe no deleterious ADE effects after infusion (27-29).
