Agreed NJ has gotten over 300,000 doses and only administered 76,000 this is on our governor to not be ready with a plan and not have his so called super vaccine centers readyYou keep saying it's a supply issue. Yet less than 20% of what has been delivered has been used. And we had the worst week for vaccinations failing to reach 3 million before the New Year. Right now supply is far down on the list of issues.
Colleges
- American Athletic
- Atlantic Coast
- Big 12
- Big East
- Big Ten
- Colonial
- Conference USA
- Independents (FBS)
- Junior College
- Mountain West
- Northeast
- Pac-12
- Patriot League
- Pioneer League
- Southeastern
- Sun Belt
- Army
- Charlotte
- East Carolina
- Florida Atlantic
- Memphis
- Navy
- North Texas
- Rice
- South Florida
- Temple
- Tulane
- Tulsa
- UAB
- UTSA
- Boston College
- California
- Clemson
- Duke
- Florida State
- Georgia Tech
- Louisville
- Miami (FL)
- North Carolina
- North Carolina State
- Pittsburgh
- Southern Methodist
- Stanford
- Syracuse
- Virginia
- Virginia Tech
- Wake Forest
- Arizona
- Arizona State
- Baylor
- Brigham Young
- Cincinnati
- Colorado
- Houston
- Iowa State
- Kansas
- Kansas State
- Oklahoma State
- TCU
- Texas Tech
- UCF
- Utah
- West Virginia
- Illinois
- Indiana
- Iowa
- Maryland
- Michigan
- Michigan State
- Minnesota
- Nebraska
- Northwestern
- Ohio State
- Oregon
- Penn State
- Purdue
- Rutgers
- UCLA
- USC
- Washington
- Wisconsin
High Schools
- Illinois HS Sports
- Indiana HS Sports
- Iowa HS Sports
- Kansas HS Sports
- Michigan HS Sports
- Minnesota HS Sports
- Missouri HS Sports
- Nebraska HS Sports
- Oklahoma HS Sports
- Texas HS Hoops
- Texas HS Sports
- Wisconsin HS Sports
- Cincinnati HS Sports
- Delaware
- Maryland HS Sports
- New Jersey HS Hoops
- New Jersey HS Sports
- NYC HS Hoops
- Ohio HS Sports
- Pennsylvania HS Sports
- Virginia HS Sports
- West Virginia HS Sports
ADVERTISEMENT
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
OT: COVID Science - Pfizer/Moderna vaccines >90% effective; Regeneron antibody cocktail looks very promising in phase II/III trial and more
- Thread starter RU848789
- Start date
- Status
- Not open for further replies.
Jeeze. Really? Why do refuse to acknowledge the real problem? No one in the US is claiming a supply problem except you.Ok, say we magically inject all 12mil doses today. Great, we’re done with those doses, but now we have to wait 2 weeks doing nothing until we get the next batch. We’re just spreading the injections out during that time period. Either way, people in group 3 ain’t getting vaccinated until the doses are there. We just have to make sure we don’t fall behind, which we really haven’t yet if you look at the CDC plan.
Also, the Bloomberg counter has been stuck at 3mil for like two days.
Here is the real problem. We need to get to nearly 2 million vaccinations A DAY, EVERYDAY. Right now supply is not the problem
What a surprise...get Romney on the case..
New York lagging behind Florida in administering coronavirus vaccines
New York lagging behind Florida in administering coronavirus vaccines
New York is lagging behind Florida in its efforts to administer the coronavirus vaccine, federal data shows, despite Gov. Andrew Cuomo and Mayor Bill de Blasio’s boasts of the Empire State…nypost.com
The Bloomberg tracker actually shows NY has used 30% of their doses compared to 22% in FL. So NY seems to be doing better.
Ash is responsible for many bad conspiracy theories.Huh? Are you saying 1000s of foreigners are streaming into the US to get vaccines? Sounds like a bad conspiracy theory.
Jeeze. Really? Why do refuse to acknowledge the real problem? No one in the US is claiming a supply problem except you.
Here is the real problem. We need to get to nearly 2 million vaccinations A DAY, EVERYDAY. Right now supply is not the problem
You realize if we injected 2 million a day, we would have run out of doses over a week ago?
I do but the foreigners weren't coming here for medical reasons. Especially for a specific reason like a vaccine.I didn't mention "vaccines" specifically but the foreigners have been bombing US for healthcare and benefits for decades now. You should know that much
Wrong as usual.What a surprise...get Romney on the case..
New York lagging behind Florida in administering coronavirus vaccines

New York lagging behind Florida in administering coronavirus vaccines
New York is lagging behind Florida in its efforts to administer the coronavirus vaccine, federal data shows, despite Gov. Andrew Cuomo and Mayor Bill de Blasio’s boasts of the Empire State…nypost.com
Even still the difference so small that he makes it into a red and blue state issue instead of focusing on the problem which is a poor federal plan.The Bloomberg tracker actually shows NY has used 30% of their doses compared to 22% in FL. So NY seems to be doing better.
You realize they aren't coming close to 1 million vaccinations in a week right?? That deliveries are exceeding vaccinations by a wide margin. Nobody has run out. Your pie in the sky theories are a waste of time.You realize if we injected 2 million a day, we would have run out of doses over a week ago?
But at least we would have 2 million vaccinated. Do you not see how wrong you are? The more now the better. And you were bad mouthing me for telling you facts. We have put ourselves behind 2-3 months just watch smart guy.You realize if we injected 2 million a day, we would have run out of doses over a week ago?
But at least we would have 2 million vaccinated. Do you not see how wrong you are? The more now the better. And you were bad mouthing me for telling you facts. We have put ourselves behind 2-3 months just watch smart guy.
I already agreed the sooner the better for each batch. Keep reading. I just think it’s way too early to say that overall, we are way behind. For example in NJ, half the doses are earmarked to nursing homes and those appointments are already scheduled and in the books. Not like this is delaying us from moving onto the next group or anything.
I thought we are currently at 3 million vaccinated? that's at least 1 million a week.You realize they aren't coming close to 1 million vaccinations in a week right?? That deliveries are exceeding vaccinations by a wide margin. Nobody has run out. Your pie in the sky theories are a waste of time.
ArminRU: we are behind and each time I mentioned the issues which were coming I had several who refused to understand we were going to be way behind . Poor planning or for a better word no competent management at the state level. Once again the buck keeps getting passed back at the Federal response which carried out the initial distribution.As the states failed the LTC issues in NY and NJ our two governors ( one an award winner) again look like amateurs. As the news has reported it will be months... many months and that is shameful.I already agreed the sooner the better for each batch. Keep reading. I just think it’s way too early to say that overall, we are way behind. For example in NJ, half the doses are earmarked to nursing homes and those appointments are already scheduled and in the books. Not like this is delaying us from moving onto the next group or anything.
Did 1918 mutate, or was it just worse in the 2nd year?
As per is it true? That's what I have read in this thread throughout this whole thing. @RU848789 ?
It took a long time and a lot of old school (finding preserved remains of infected people from 1918, especially soldiers) and new school (viral sequencing and testing in animals), but by about 10 years ago it was finally determined that there were mutations in the H1N1 virus that caused the 1918 pandemic did, indeed, mutate over the course of the pandemic. The mutations appear to have mostly made the virus more transmissible, not necessarily more lethal, but with very little medical interventions back then, the more people infected, the more who were likely to die, especially in the midst of a World War, with lots of wounded soldiers and tons of movements of soldiers and support staff. Secondary bacterial infections also played a major role in deaths (which would not happen today with antibiotics).
https://www.thelancet.com/journals/lanres/article/PIIS2213-2600(18)30272-8/fulltext
Research into the 1918 virus was reinvigorated in 1997 by the elucidation of its sequence, pieced together from fragments amplified from histological sections of lungs of soldiers who died of the disease. This enabled the reconstitution of the virus from synthetic complementary DNAs, and studies in animal models revealed a pathogenicity higher than that seen after experimental infection with any modern day seasonal influenza viruses. These advances coincided with the emergence of a new highly pathogenic virus (avian influenza A H5N1), that crossed over into people exposed to infected poultry in 1997, and again in 2003, sparking great concern that it might initiate a new pandemic. Then, the 2009 H1N1 swine flu pandemic struck, triggering a wave of genomic and immunological research that described the human response to influenza at a level not possible during previous outbreaks and defined clear risk factors for serious disease.
Influenza pandemics arise when a virus with novel antigenicity acquires the ability to transmit between people. These viruses originate from animal reservoirs, typically birds or pigs. For human-to-human transmission to be maintained, the major surface antigen (viral haemagglutinin, HA) has to be able to bind to receptors in the human airway and to be chemically stable enough to survive between human hosts in airborne droplets.
A series of autopsy cases of soldiers who died from influenza in 1918 reveal this evolutionary process; the viral sequences obtained from the lungs of victims who died in May 1918 (before the pandemic really took off), show an HA that binds avian-like receptors and confers poor airborne transmissibility between ferrets. However, by autumn 1918, the autopsy material reveals that the virus had mutated in ways that enhanced its ability to bind human airway receptors, presumably gaining transmissibility. Similar studies of the 2009 H1N1 pandemic virus also showed the transition from a first wave virus only just adapted enough to sustain transmission, to a third wave virus that had potentiated its adaptation to its new host.
Although the 1918 influenza virus is especially virulent in cells and experimental animal models, a strong body of evidence implicates other pathogens in the extreme loss of life of the pandemic: most of the human victims were co-infected with bacteria such as Streptococcus pneumoniae, Strep. pyogenes or Staphylococcus aureus. Mice co-infected with 1918 influenza virus and Strep. pneumoniae show enhanced disease characterised by a neutrophil-driven transcriptomic signature and histological evidence of coagulation and pulmonary thrombosis reminiscent of autopsy slides from human 1918 cases.
I already agreed the sooner the better for each batch. Keep reading. I just think it’s way too early to say that overall, we are way behind. For example in NJ, half the doses are earmarked to nursing homes and those appointments are already scheduled and in the books. Not like this is delaying us from moving onto the next group or anything.
Thank you for your logical take and approach. Unfortunately you are stuck with unqualified individuals who perpetually criticize everything. The fact we even have a vaccine, much less multiple, now is an incredible achievement. But some people need immediate gratification and don’t have any respect for the actual process which is pain staking and endless hours of work.
Everyone agrees that the vaccine development is a wild success beyond what anyone would have hoped. That doesn’t mean everything else involved, which is quite a lot, couldn't also be done with equal zeal and success.Thank you for your logical take and approach. Unfortunately you are stuck with unqualified individuals who perpetually criticize everything. The fact we even have a vaccine, much less multiple, now is an incredible achievement. But some people need immediate gratification and don’t have any respect for the actual process which is pain staking and endless hours of work.
Of course not, as I know you know. I'm also guessing you saw the recent breakthroughs on cryogenic electron microscopy and its application to being able to essentially "see" down to just about the molecular level for the proteins, potentially obviating the need to grow pure single crystals of proteins and even small molecules in order to determine structure, which historically has been done via X-ray diffraction patterns on such crystals. Derek Lowe posted on this yesterday on his blog, linked below, as two breakthrough papers on this technology were just published in Nature.
Crystals are very precisely ordered/arranged and the diffraction pattern can be analyzed via software to determine exactly how molecules are connected and crystals are usually fairly easy to isolate for small molecules (although not always - I've done a ton of work in the field of crystallography and have 2 patents in this area). However, it's often extremely hard to grow pure crystals of biological molecules like proteins, so their structure can be elucidated by X-rays. But with cryo electron microscopy, it may not always be necessary any more as this technology can elucidate 3-D structure directly (via very advanced software).
https://blogs.sciencemag.org/pipeline/archives/2020/10/30/down-to-the-atoms
https://www.nature.com/articles/s41586-020-2829-0
https://www.nature.com/articles/s41586-020-2833-4
In addition, this technology has been put to work to help elucidate some of the structural elements of the proteins on the SARS-CoV-2 virus and how they interact with various human proteins - in particular the peptidases of the angiotensin-converting enzyme 2 (ACE2), which the virus "mates with" via its spike proteins to gain entry into various epithelial cells in the lungs and blood vessels and elsewhere. The graphic below shows the outcome of some of this work - pretty cool stuff.
https://directorsblog.nih.gov/tag/cryo-em/
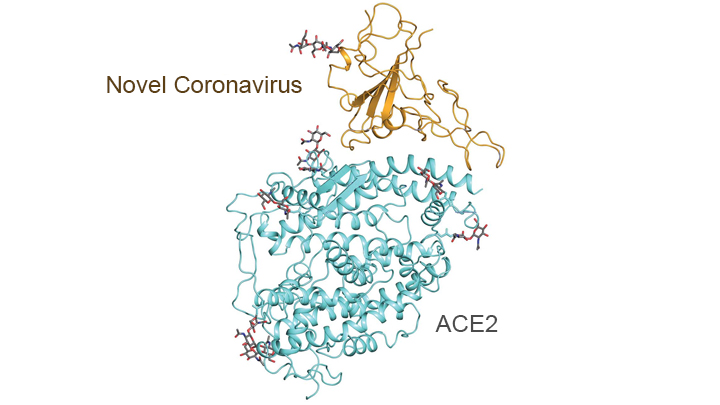
Thought this article that just came out in NatGeo did a really nice job of elaborating on some of the work discussed in the post above, telling at least part of the story of the last 12+ years of R&D into elucidating virus structures and how that paved the way for developing the mRNA vaccines for SARS-CoV-2. The work started with trying to define the protein "shape" (proteins are 3-dimensional and can exist in numerous conformations, which can look quite different) of the respiratory syncytial virus (RSV, one of the common cold viruses), wherein they eventually were able to isolate single crystals of the viral "fusion protein" revealing its shape and suggesting how one might stabilize that shape by addition of a couple of amino acids - this worked very well and the stabilized version of the fusion protein elicited a much more robust antigenic response for RSV.
Fast forward to about 2015 and some of the same folks who did the work above were involved in working with the betacoronavirus that causes MERS (similar to SARS and SARS-CoV-2; RSV is not a betacoronavirus), trying to figure out its spike protein shape. Along came another improvement in viral protein structure elucidation, discussed at length in the post above: cryogenic electron microscopy, which slows all motion down to near zero at supercold temps, allowing "pictures" to be taken of protein shapes - this was necessary for betacoronaviruses, which are much harder to crystallize than RSV.
Again, knowing the 3-D structure allowed these scientists to figure out how to stabilize that protein structure, which allowed development of an mRNA vaccine for MERS, which they co-developed with Moderna and which worked in animals, but was never tested in humans, since the outbreak dieed out. Of course, having done all that fundamental work on protein imaging, structures and stabilization and application of that to developing an mRNA vaccine against MERS, made the rapid development and testing of Moderna's mRNA vaccine for SARS-CoV-2 possible - and the other vaccines all utilized that fundamental research in designing their vaccines.
https://www.nationalgeographic.com/...20210101&rid=3F7A7D00850AD922736B3173646A296D
Excellent and important new paper from La Jolla Institute of Immunology by Shane Crotty's group, which has been the subject of a few posts by me and @UMRU and others over the last several months. Essentially, they followed over 180 recovered infected patients for 5-8 months, performing the most comprehensive assessment of ongoing immunological marker levels in patients, by profiling antibodies, B-cells, and T-cells in their immune systems over time.
They found durable responses for the vast majority of people and have postulated that immunity in these people could very well last for years and it's expected that immunity from vaccines would likely be similar - see the excerpt below from the Times article (the paper is in the 2nd link), especially the part in bold. This work builds on the work done by many others around the world in recent months (some of which is in the 3rd/4th links from old posts of mine).
https://www.nytimes.com/2020/11/17/health/coronavirus-immunity.html/??
https://www.biorxiv.org/content/10.1101/2020.11.15.383323v1.full.pdf
https://rutgers.forums.rivals.com/threads/florida-halts-football-program-covid.202128/post-4726680
https://rutgers.forums.rivals.com/t...es-interventions-and-more.198855/post-4650144
How long might immunity to the coronavirus last? Years, maybe even decades, according to a new study — the most hopeful answer yet to a question that has shadowed plans for widespread vaccination.
Eight months after infection, most people who have recovered still have enough immune cells to fend off the virus and prevent illness, the new data show. A slow rate of decline in the short term suggests, happily, that these cells may persist in the body for a very, very long time to come.
The research, published online, has not been peer-reviewed nor published in a scientific journal. But it is the most comprehensive and long-ranging study of immune memory to the coronavirus to date.
“That amount of memory would likely prevent the vast majority of people from getting hospitalized disease, severe disease, for many years,” said Shane Crotty, a virologist at the La Jolla Institute of Immunology who co-led the new study.
Update to the duration of immune responses in infected/recovered cases from the original outbreak in Wuhan, where they now have up to 9-months of data showing the vast majority of recovered COVID patients still have durable memory T-cell immune, which bodes well for longer term immunity for these people and especially for vaccinated people as the vaccines usually raise a more robust immune response than infections do.
https://www.biorxiv.org/content/10.1101/2020.11.15.383463v1.full
Magnitude and breadth of SARS-CoV-2 memory CD4 and CD8 T cell responses were heterogeneous between patients but robust responses could be detected up to 9 months post disease onset in most convalescent individuals.
One more COVID science post tonight. Came across this COVID Fact Sheet from the Belgian CDC, which I think is the single best, most comprehensive collection of useful info I have seen to date and each entry also contains references and links for many topics, plus all the most recent entries are in green for easy identification. Below is the Index for the document, showing all the topics that are discussed in pretty decent depth in this 57 page document. Enjoy.
https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_fact_sheet_ENG.pdf

https://covid-19.sciensano.be/sites/default/files/Covid19/COVID-19_fact_sheet_ENG.pdf

Saw an article where England is having a spike in the need to hospitalize children with Covid
Covid wards 'full of children' for first time in pandemic, warn nurses
By Patrick Sawer
telegraph.co.uk — Medics are starting to see “whole wards of children” suffering from Covid for the first time during the pandemic, a senior nurse has warned. Laura Duffell, a matron at King’s College Hospital, London, said the new strain of Covid was affecting children and younger adults with no underlying health conditions in worrying numbers. She said: “It’s very different. That’s what makes it so much scarier for us as doctors, nurses and porters and everyone else who is working on the front line.
Covid wards 'full of children' for first time in pandemic, warn nurses
By Patrick Sawer
telegraph.co.uk — Medics are starting to see “whole wards of children” suffering from Covid for the first time during the pandemic, a senior nurse has warned. Laura Duffell, a matron at King’s College Hospital, London, said the new strain of Covid was affecting children and younger adults with no underlying health conditions in worrying numbers. She said: “It’s very different. That’s what makes it so much scarier for us as doctors, nurses and porters and everyone else who is working on the front line.
Saw an article where England is having a spike in the need to hospitalize children with Covid
Covid wards 'full of children' for first time in pandemic, warn nurses
By Patrick Sawer
telegraph.co.uk — Medics are starting to see “whole wards of children” suffering from Covid for the first time during the pandemic, a senior nurse has warned. Laura Duffell, a matron at King’s College Hospital, London, said the new strain of Covid was affecting children and younger adults with no underlying health conditions in worrying numbers. She said: “It’s very different. That’s what makes it so much scarier for us as doctors, nurses and porters and everyone else who is working on the front line.
Unfortunate. This may prolong lockdowns both government and self administered.
Update to the duration of immune responses in infected/recovered cases from the original outbreak in Wuhan, where they now have up to 9-months of data showing the vast majority of recovered COVID patients still have durable memory T-cell immune, which bodes well for longer term immunity for these people and especially for vaccinated people as the vaccines usually raise a more robust immune response than infections do.
https://www.biorxiv.org/content/10.1101/2020.11.15.383463v1.full
Magnitude and breadth of SARS-CoV-2 memory CD4 and CD8 T cell responses were heterogeneous between patients but robust responses could be detected up to 9 months post disease onset in most convalescent individuals.
Another pre-print pointing to long lasting immunity.
They should encourage people who were already infected to get in the back of the line, especially those under 50.
SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans
Infection or vaccination induces a population of long-lived bone marrow plasma cells (BMPCs) that are a persistent and essential source of protective antibodies1–5. Whether this population is induced in patients infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is un...
Ya, but I'd be surprised if those that have had it are in line at all at this point.Another pre-print pointing to long lasting immunity.
They should encourage people who were already infected to get in the back of the line, especially those under 50.
SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans
Infection or vaccination induces a population of long-lived bone marrow plasma cells (BMPCs) that are a persistent and essential source of protective antibodies1–5. Whether this population is induced in patients infected with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is un...www.researchsquare.com
Saw an article where England is having a spike in the need to hospitalize children with Covid
Covid wards 'full of children' for first time in pandemic, warn nurses
By Patrick Sawer
telegraph.co.uk — Medics are starting to see “whole wards of children” suffering from Covid for the first time during the pandemic, a senior nurse has warned. Laura Duffell, a matron at King’s College Hospital, London, said the new strain of Covid was affecting children and younger adults with no underlying health conditions in worrying numbers. She said: “It’s very different. That’s what makes it so much scarier for us as doctors, nurses and porters and everyone else who is working on the front line.
Looks like that report was a little over done

Coronavirus: No increase in severe child cases, paediatricians say
"Covid is rife in hospitals, but not among children," consultant paediatrician Dr Ronny Cheung said.
Looks like that report was a little over done

Coronavirus: No increase in severe child cases, paediatricians say
"Covid is rife in hospitals, but not among children," consultant paediatrician Dr Ronny Cheung said.www.bbc.com
Good news
Looks like that report was a little over done

Coronavirus: No increase in severe child cases, paediatricians say
"Covid is rife in hospitals, but not among children," consultant paediatrician Dr Ronny Cheung said.www.bbc.com
What a dumbass that nurse is.
Could have told you that when the first coverage broke. Anything to place more fears and concerns in the people. What’s the excuse for these news stories anyway. Time to stop and get the vaccine distributed starting with those in care facilities.Looks like that report was a little over done

Coronavirus: No increase in severe child cases, paediatricians say
"Covid is rife in hospitals, but not among children," consultant paediatrician Dr Ronny Cheung said.www.bbc.com
Looks like that report was a little over done

Coronavirus: No increase in severe child cases, paediatricians say
"Covid is rife in hospitals, but not among children," consultant paediatrician Dr Ronny Cheung said.www.bbc.com
As i thought
Another issue for the northeast will be the weather predictions being listed again for the next several months SNOW. Even if the vaccine is available storms and weather conditions will impact the distribution to the public. Nobody is figuring this into the equation of vaccinating people ? Will impact many in the over 70 -80’s groups.
If this really works, why wasn't it in the original rollout plan?
 www.cnbc.com
www.cnbc.com

U.S. could ramp up slow Covid vaccine rollout by giving two half volume doses of Moderna shot, Slaoui says
Moncef Slaoui told CBS that Operation Warp Speed is looking at giving half doses of the Moderna vaccine to certain groups to speed up the Covid vaccine rollout.
Well maybe it works but what happens when the 2nd half dose isn’t available .What is the timeline between injections before it would be obsolete? Only would be done to quiet those who feel they will not be able to get the vaccine quickly and safely. Can see this becoming more of a fiasco over the next several months with some getting a full dosage and others being left behind . Which groups or segments are less valued? I would wager they already have determined that part in the process.
There will be no problem with the second dose as being availableWell maybe it works but what happens when the 2nd half dose isn’t available .What is the timeline between injections before it would be obsolete? Only would be done to quiet those who feel they will not be able to get the vaccine quickly and safely. Can see this becoming more of a fiasco over the next several months with some getting a full dosage and others being left behind . Which groups or segments are less valued? I would wager they already have determined that part in the process.
Coronavirus outbreak in California emergency room infects 43 hospital staffers
Dozens of emergency room staff members at a hospital in San Jose, Calif., tested positive for coronavirus last week in an outbreak that may have been spread by an employee who wore an "air-powered costume" in the department on Christmas Day, hospital officials said.
Chavez said one of the infected staffers had appeared briefly in the department wearing an inflatable costume to spread cheer during the holidays, the station reported.
"Any exposure, if it occurred, would have been completely innocent, and quite accidental, as the individual had no COVID symptoms and only sought to lift the spirits of those around them during what is a very stressful time," the hospital said in the statement.
If this really works, why wasn't it in the original rollout plan?

U.S. could ramp up slow Covid vaccine rollout by giving two half volume doses of Moderna shot, Slaoui says
Moncef Slaoui told CBS that Operation Warp Speed is looking at giving half doses of the Moderna vaccine to certain groups to speed up the Covid vaccine rollout.www.cnbc.com
Interesting idea. I remember the responses being fairly similar in some of the different dose sizes during early testing. Only problem is that there isn’t much testing to support it, but on paper it should work.
Oh like the first series of this current vaccination cycle. Where do you see having enough not only here but every other state that would use the Moderna vax. Very short sighted in that type of thinking. Still the question what is the timeline between the 1/2 dose shots? 3 months, 6 months?There will be no problem with the second dose as being available
Last edited:
Saw an article where England is having a spike in the need to hospitalize children with Covid
Covid wards 'full of children' for first time in pandemic, warn nurses
By Patrick Sawer
telegraph.co.uk — Medics are starting to see “whole wards of children” suffering from Covid for the first time during the pandemic, a senior nurse has warned. Laura Duffell, a matron at King’s College Hospital, London, said the new strain of Covid was affecting children and younger adults with no underlying health conditions in worrying numbers. She said: “It’s very different. That’s what makes it so much scarier for us as doctors, nurses and porters and everyone else who is working on the front line.
Turned out to be Fake News
Turned out to be Fake News
Was it fake or did a nurse see an anecdote and expand its meaning?
That is fairly common
Whether deliberate or unintentional, it's still fake news and harmful.Was it fake or did a nurse see an anecdote and expand its meaning?
That is fairly common
No incorrect news that was corrected.Turned out to be Fake News
Fake connotes made up, it’s not made up. It’s poor reporting that was corrected by better reporting. They shouldn’t have relied on one person POV however first hand it is. Particularly with a matter as important as this.Whether deliberate or unintentional, it's still fake news and harmful.
I would edit this and say that fake connotes intentional deception.
Last edited:
- Status
- Not open for further replies.
Similar threads
ADVERTISEMENT
ADVERTISEMENT