Don't mention it!
Anytime Sanu
Don't mention it!
Evidence looks pretty strong here. Even stronger in the article.Interesting article written by Steven Hatfill, a veteran virologist who helped establish the Rapid Hemorrhagic Fever Response Teams for the National Medical Disaster Unit in Kenya, Africa. He is an adjunct assistant professor in two departments at the George Washington University Medical Center where he teaches mass casualty medicine.
Hoping for concise responses--in layman's terms--from the pharma/medical-trained posters here to the highlights of this article absent political views. Thanks.
"HCQ and the Fauci Strategy"
So what is the real story on hydroxychloroquine? Here, briefly, is what we know:
When the COVID-19 pandemic began, a search was made for suitable antiviral therapies to use as treatment until a vaccine could be produced. One drug, hydroxychloroquine, was found to be the most effective and safe for use against the virus. Federal funds were used for clinical trials of it, but there was no guidance from Dr. Anthony Fauci or the NIH Treatment Guidelines Panel on what role the drug would play in the national pandemic response. Fauci seemed to be unaware that there actually was a national pandemic plan for respiratory viruses.
Following a careful regimen developed by doctors in France, some knowledgeable practicing U.S. physicians began prescribing hydroxychloroquine to patients still in the early phase of COVID infection. Its effects seemed dramatic. Patients still became sick, but for the most part they avoided hospitalization. In contrast --- and in error -- the NIH-funded studies somehow became focused on giving hydroxychloroquine to late-presenting hospitalized patients. This was in spite of the fact that unlike the drug’s early use in ambulatory patients, there was no real data to support the drug’s use in more severe hospitalized patients.
On April 6, 2020, an international team of medical experts published an extensive study of hydroxychloroquine in more than 130,000 patients with connective tissue disorders. They reaffirmed that hydroxychloroquine was a safe drug with no serious side effects. The drug could safely be given to pregnant women and breast-feeding mothers. Consequently, countries such as China, Turkey, South Korea, India, Morocco, Algeria, and others began to use hydroxychloroquine widely and early in their national pandemic response. Doctors overseas were safely prescribing the drug based on clinical signs and symptoms because widespread testing was not available.
However, the NIH promoted a much different strategy for the United States. The “Fauci Strategy” was to keep early infected patients quarantined at home without treatment until they developed a shortness of breath and had to be admitted to a hospital. Then they would they be given hydroxychloroquine. The Food and Drug Administration cluelessly agreed to this doctrine and it stated in its hydroxychloroquine Emergency Use Authorization (EUA) that “hospitalized patients were likely to have a greater prospect of benefit (compared to ambulatory patients with mild illness).”
At the same time, accumulating data showed remarkable results if hydroxychloroquine were given to patients early, during a seven-day window from the time of first symptom onset. If given during this window, most infections did not progress into the severe, lethal second stage of the disease. Patients still got sick, but they avoided hospitalization or the later transfer to an intensive care unit. In mid-April a high-level memo was sent to the FDA alerting them to the fact that the best use for hydroxychloroquine was for its early use in still ambulatory COVID patients. These patients were quarantined at home but were not short of breath and did not yet require supplemental oxygen and hospitalization.
Failing to understand that COVID-19 could be a two-stage disease process, the FDA ignored the memo and, as previously mentioned, it withdrew its EUA for hydroxychloroquine based on flawed studies and clinical trials that were applicable only to late-stage COVID patients.
By now, however, some countries had already implemented early, aggressive, outpatient community treatment with hydroxychloroquine and within weeks were able to minimize their COVID deaths and bring their national pandemic under some degree of control.
In countries such as Great Britain and the United States, where the “Fauci-Hahn Strategy” was followed, there was a much higher death rate and an ever-increasing number of cases. COVID patients in the U.S. would continue to be quarantined at home and left untreated until they developed shortness of breath. Then they would be admitted to the hospital and given hydroxychloroquine outside the narrow window for the drug’s maximum effectiveness.
In further contrast, countries that started out with the “Fauci-Hahn Doctrine” and then later shifted their policy towards aggressive outpatient hydroxychloroquine use, after a brief lag period also saw a stunning rapid reduction in COVID mortality and hospital admissions.
There are now 53 studies that show positive results of hydroxychloroquine in COVID infections. There are 14 global studies that show neutral or negative results -- and 10 of them were of patients in very late stages of COVID-19, where no antiviral drug can be expected to have much effect. Of the remaining four studies, two come from the same University of Minnesota author. The other two are from the faulty Brazil paper, which should be retracted, and the fake Lancet paper, which was.
https://www.realclearpolitics.com/a...t_the_media_continues_to_besmirch_143875.html
Excellent information in this article. If true Let’s get this rolling.Interesting article written by Steven Hatfill, a veteran virologist who helped establish the Rapid Hemorrhagic Fever Response Teams for the National Medical Disaster Unit in Kenya, Africa. He is an adjunct assistant professor in two departments at the George Washington University Medical Center where he teaches mass casualty medicine.
Hoping for concise responses--in layman's terms--from the pharma/medical-trained posters here to the highlights of this article absent political views. Thanks.
"HCQ and the Fauci Strategy"
So what is the real story on hydroxychloroquine? Here, briefly, is what we know:
When the COVID-19 pandemic began, a search was made for suitable antiviral therapies to use as treatment until a vaccine could be produced. One drug, hydroxychloroquine, was found to be the most effective and safe for use against the virus. Federal funds were used for clinical trials of it, but there was no guidance from Dr. Anthony Fauci or the NIH Treatment Guidelines Panel on what role the drug would play in the national pandemic response. Fauci seemed to be unaware that there actually was a national pandemic plan for respiratory viruses.
Following a careful regimen developed by doctors in France, some knowledgeable practicing U.S. physicians began prescribing hydroxychloroquine to patients still in the early phase of COVID infection. Its effects seemed dramatic. Patients still became sick, but for the most part they avoided hospitalization. In contrast --- and in error -- the NIH-funded studies somehow became focused on giving hydroxychloroquine to late-presenting hospitalized patients. This was in spite of the fact that unlike the drug’s early use in ambulatory patients, there was no real data to support the drug’s use in more severe hospitalized patients.
On April 6, 2020, an international team of medical experts published an extensive study of hydroxychloroquine in more than 130,000 patients with connective tissue disorders. They reaffirmed that hydroxychloroquine was a safe drug with no serious side effects. The drug could safely be given to pregnant women and breast-feeding mothers. Consequently, countries such as China, Turkey, South Korea, India, Morocco, Algeria, and others began to use hydroxychloroquine widely and early in their national pandemic response. Doctors overseas were safely prescribing the drug based on clinical signs and symptoms because widespread testing was not available.
However, the NIH promoted a much different strategy for the United States. The “Fauci Strategy” was to keep early infected patients quarantined at home without treatment until they developed a shortness of breath and had to be admitted to a hospital. Then they would they be given hydroxychloroquine. The Food and Drug Administration cluelessly agreed to this doctrine and it stated in its hydroxychloroquine Emergency Use Authorization (EUA) that “hospitalized patients were likely to have a greater prospect of benefit (compared to ambulatory patients with mild illness).”
At the same time, accumulating data showed remarkable results if hydroxychloroquine were given to patients early, during a seven-day window from the time of first symptom onset. If given during this window, most infections did not progress into the severe, lethal second stage of the disease. Patients still got sick, but they avoided hospitalization or the later transfer to an intensive care unit. In mid-April a high-level memo was sent to the FDA alerting them to the fact that the best use for hydroxychloroquine was for its early use in still ambulatory COVID patients. These patients were quarantined at home but were not short of breath and did not yet require supplemental oxygen and hospitalization.
Failing to understand that COVID-19 could be a two-stage disease process, the FDA ignored the memo and, as previously mentioned, it withdrew its EUA for hydroxychloroquine based on flawed studies and clinical trials that were applicable only to late-stage COVID patients.
By now, however, some countries had already implemented early, aggressive, outpatient community treatment with hydroxychloroquine and within weeks were able to minimize their COVID deaths and bring their national pandemic under some degree of control.
In countries such as Great Britain and the United States, where the “Fauci-Hahn Strategy” was followed, there was a much higher death rate and an ever-increasing number of cases. COVID patients in the U.S. would continue to be quarantined at home and left untreated until they developed shortness of breath. Then they would be admitted to the hospital and given hydroxychloroquine outside the narrow window for the drug’s maximum effectiveness.
In further contrast, countries that started out with the “Fauci-Hahn Doctrine” and then later shifted their policy towards aggressive outpatient hydroxychloroquine use, after a brief lag period also saw a stunning rapid reduction in COVID mortality and hospital admissions.
There are now 53 studies that show positive results of hydroxychloroquine in COVID infections. There are 14 global studies that show neutral or negative results -- and 10 of them were of patients in very late stages of COVID-19, where no antiviral drug can be expected to have much effect. Of the remaining four studies, two come from the same University of Minnesota author. The other two are from the faulty Brazil paper, which should be retracted, and the fake Lancet paper, which was.
https://www.realclearpolitics.com/a...t_the_media_continues_to_besmirch_143875.html
If true Fauci may have been the worst possible person at the worst possible time heading our National Institute of Allergy and Infectious Diseases (NIAID), sadly.Evidence looks pretty strong here. Even stronger in the article.
Would like to hear a rebuttal, if there is one.Excellent information in this article. If true Let’s get this rolling.
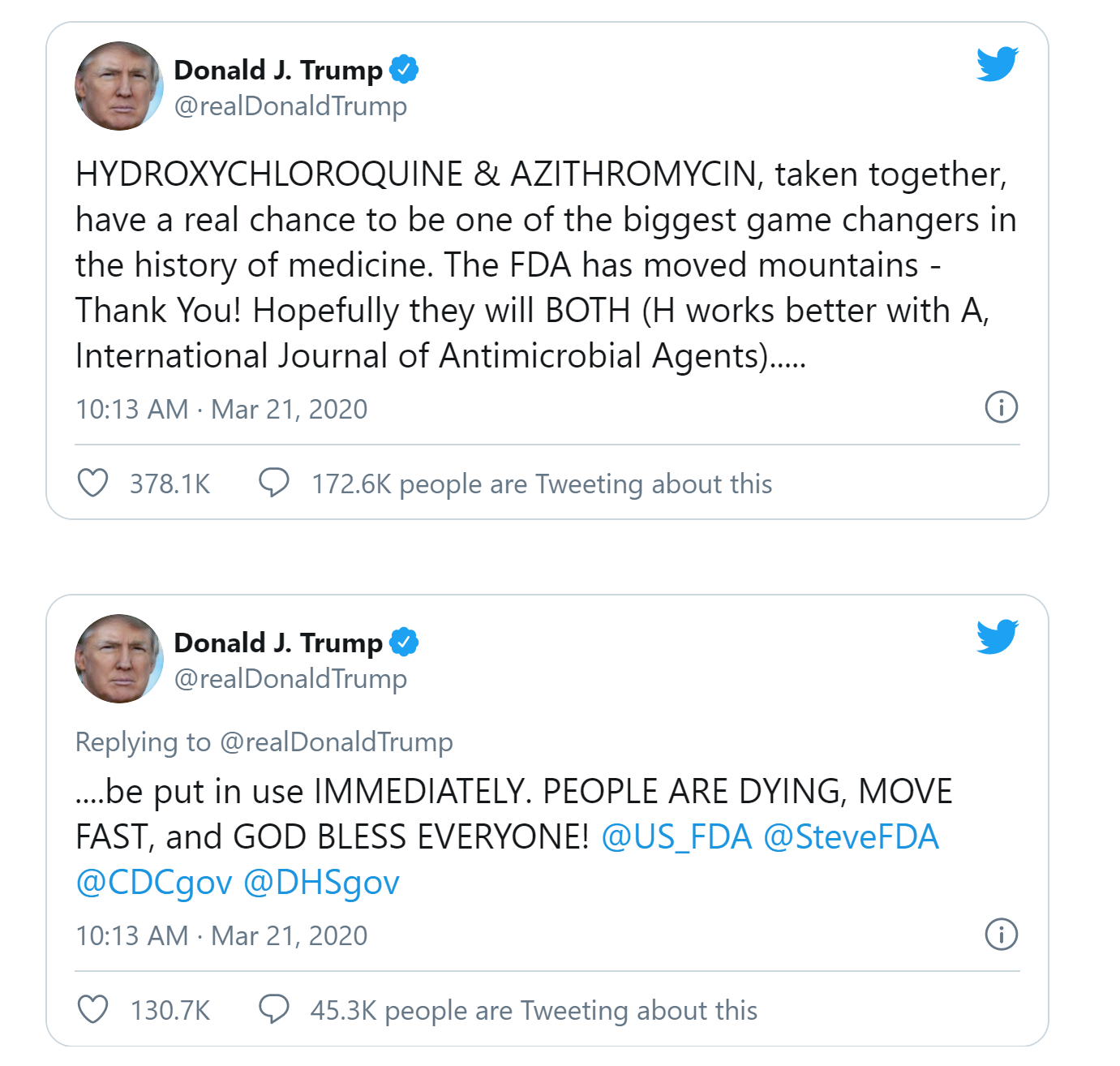
Are you kidding me?Do you really think people are so gullible as to believe your post here? People remember the many things Trump said about HCQ, but it all started with these tweets, which were far beyond, "let's give it a shot." And even since then, he's made countless statements, like "try it, what have you got to lose?" and even going so far as to tweet the story of that nutty alien demon seed doctor touting HCQ/Az/Zn. The outcome of all this hype was that 483,000 mor prescriptions for HCQ were written by doctors during the 10-week period between Feb. 17 and April 27 (vs. the same time period in 2019).
https://theconversation.com/when-tr...despite-little-evidence-that-it-worked-140156
I agree, many mistakes/errors have been made, starting in China, at the WHO, and yes at our federal government, plus at the state/local levels in the U.S., that would have lessened the terrible consequences of this global natural disaster.The "let's pin all the fault on this guy" game is just not going to lead to a good discussion.
You sure it’s natural?I agree, many mistakes/errors have been made, starting in China, at the WHO, and yes at our federal government, plus at the state/local levels in the U.S., that would have lessened the terrible consequences of this global natural disaster.
fair point...I'll assume it is, for nowYou sure it’s natural?
I'm reminded of the great philosopher Mike Tyson who once said "Everyone has a plan 'till they get punched in the mouth".The "let's pin all the fault on this guy" game is just not going to lead to a good discussion.

Ya, and sometimes you need to get punched in the face to know you are actually in a fight.I'm reminded of the great philosopher Mike Tyson who once said "Everyone has a plan 'till they get punched in the mouth".

I'd say it was more like a Hulk-sized roundhouse sucker punch here.Ya, and sometimes you need to get punched in the face to know you are actually in a fight.
Who said anything about the drug being 100% effective?Here is the article I referred to earlier
https://news.yahoo.com/sorry-burst-magic-bubble-says-180317285.html
Michigan Democratic lawmaker says hydroxychloroquine saved her lifeHere is the article I referred to earlier
https://news.yahoo.com/sorry-burst-magic-bubble-says-180317285.html
Michigan Democratic lawmaker says hydroxychloroquine saved her life
“If President Trump had not talked about this, it wouldn’t have been something that would be accessible for anyone to be able to get right now,” the lawmaker said
https://nypost.com/2020/04/07/michigan-democrat-says-hydroxychloroquine-saved-her-life/
Who said anything about the drug being 100% effective?
You missed my point, you're pushing the narrative that based on one case, it won't work.So how effective is it supposed to be 1%, 10%, 50%?
Those who say it work what does the data show as how effective?
If it is not at least above 50% effective is it really a treatment?
If the percentage is significantly below 50% can it just be that those people would have improved without the drug?
No I am notYou missed my point, you're pushing the narrative that based on one case, it won't work.
My wife has mixed connective tissue disease and there are various treatments but frequently they work for a while and then stop working. My wife and her doctor are discussing what to try next and in the mean time she went back on Plaquenil while they decide. The doctor said with Covid and the fact she had done okay on it in the past it made sense to take it at this time.Wake me up when HCQ shows something in a legit trial. I gave up on it after my doctor said it doesn’t do anything.
There are so many other treatments currently in trial that have much more hope than HCQ, wish people would focus on that stuff (thank god the scientists are).
My wife has mixed connective tissue disease and there are various treatments but frequently they work for a while and then stop working. My wife and her doctor are discussing what to try next and in the mean time she went back on Plaquenil while they decide. The doctor said with Covid and the fact she had done okay on it in the past it made sense to take it at this time.
My wife went back on it when it was initially brought up as a treatment. She really is going to ultimately go back on a different drug for her illnessYeah I had like an hour conversation with my doctor when I went in for my routine checkup a few weeks ago. Since his practice was basically shut, he spent all his time at the hospital, in the front lines during it all. He said HCQ didn’t do much of anything, Remdesivir helped a little, and that they’ve been giving steroids for awhile (well before that study made the news). He eventually stopped giving HCQ after he saw the failed trials and from personal experience (had many patients die). This is all anecdotal stuff at the end of the day....who knows, maybe it works a little.
Somebody on the Joe Rogan podcast was pushing a Yale Univ Public Health article that I have been unable to find that shows that Hydroxi serves a useful purpose as an effective prophylactic and can be very useful in treatment during the early stages of COVID.
I don't think this makes Trump a genius or puts RU#'s reputation in jeopardy.
I think it means that we are still at the early stages of understanding this virus and we don't know more than we do and that applies to everyone; Trump, Fauci, Birx etc.
In the early days of HIV/AIDS, it was the same. People thought you could get it by being in the same room. Nurses refused to treat HIV/AIDS patients and blood banks were using positive patients blood to the general population.
Let's all try and be open-minded regardless of what the source is and be a little more forgiving.
Hope y'all are playing nicely lol, still no power here and spent an hour getting a generator working so we could save all our food and then most of the day chainsawing some huge branches and cleaning up the yard... what a mess, but at least no damage. Hope everyone made it through the storm ok...
YES YES YES Bravisimo
Damn fine article. Thanks for posting! You are the Common Sense King of this thread!
13 lbs? Probably up to 30+ lbs by now!Hope that 13 lbs of hoarded grind meat makes it.:Laughing


Another big company taking the free money.Johnson & Johnson reaches deal with U.S. for 100 million doses of vaccine
Johnson & Johnson announced that it will develop and deliver 100 million doses of its coronavirus vaccine for the U.S. in a deal totaling more than $1 billion, according to a statement. J&J’s vaccine candidate, Ad26.COV2.S, is expected to begin late-stage human trials ahead of schedule in September, company executives have previously predicted.
“We are scaling up production in the U.S. and worldwide to deliver a SARS-CoV-2 vaccine for emergency use,” said Dr. Paul Stoffels, chief scientific officer at Johnson & Johnson, in a statement.
The U.S. government had previously awarded J&J $456 million to develop its vaccine in collaboration with the Biomedical Advanced Research and Development Authority, or BARDA. The company said its goal is to supply more than one billion doses globally in 2021. — Noah Higgins-Dunn
Another pharma company working to save the world. Including you.Another big company taking the free money.