They considered that a mostly peaceful protest. It’s all good. Covid doesn’t work at those! In fact, I don’t know why we are even looking for a cure now. We’ve already found it!I am waiting for the spike from the 4th of July celebration in South Dakota from the open air outdoor event with no masks..oh wait nevermind
Colleges
- American Athletic
- Atlantic Coast
- Big 12
- Big East
- Big Ten
- Colonial
- Conference USA
- Independents (FBS)
- Junior College
- Mountain West
- Northeast
- Pac-12
- Patriot League
- Pioneer League
- Southeastern
- Sun Belt
- Army
- Charlotte
- East Carolina
- Florida Atlantic
- Memphis
- Navy
- North Texas
- Rice
- South Florida
- Temple
- Tulane
- Tulsa
- UAB
- UTSA
- Boston College
- California
- Clemson
- Duke
- Florida State
- Georgia Tech
- Louisville
- Miami (FL)
- North Carolina
- North Carolina State
- Pittsburgh
- Southern Methodist
- Stanford
- Syracuse
- Virginia
- Virginia Tech
- Wake Forest
- Arizona
- Arizona State
- Baylor
- Brigham Young
- Cincinnati
- Colorado
- Houston
- Iowa State
- Kansas
- Kansas State
- Oklahoma State
- TCU
- Texas Tech
- UCF
- Utah
- West Virginia
- Illinois
- Indiana
- Iowa
- Maryland
- Michigan
- Michigan State
- Minnesota
- Nebraska
- Northwestern
- Ohio State
- Oregon
- Penn State
- Purdue
- Rutgers
- UCLA
- USC
- Washington
- Wisconsin
High Schools
- Illinois HS Sports
- Indiana HS Sports
- Iowa HS Sports
- Kansas HS Sports
- Michigan HS Sports
- Minnesota HS Sports
- Missouri HS Sports
- Nebraska HS Sports
- Oklahoma HS Sports
- Texas HS Hoops
- Texas HS Sports
- Wisconsin HS Sports
- Cincinnati HS Sports
- Delaware
- Maryland HS Sports
- New Jersey HS Hoops
- New Jersey HS Sports
- NYC HS Hoops
- Ohio HS Sports
- Pennsylvania HS Sports
- Virginia HS Sports
- West Virginia HS Sports
ADVERTISEMENT
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
COVID-19 Pandemic: Transmissions, Deaths, Treatments, Vaccines, Interventions and More...
- Thread starter Richie O
- Start date
- Status
- Not open for further replies.
From what I read, a major concern about Sturgis was that the indoor bars and restaurants in the town were open without restrictions / masks.
And....its called living. They are living in Switzerland too.
Hopeful that perhaps governments and government entities take a fresh look at what the diet and food recommendations have done to obesity this country. Still wondering why a hospital serves patients toast, cereal and orange juice for breakfast.

 www.nbcnewyork.com
www.nbcnewyork.com

Doctors Studying Why Obesity May Be Tied to Serious COVID-19
Obesity seems to put people at higher risk for getting very sick if they’re infected with the coronavirus, and doctors are trying to figure out why
 www.nbcnewyork.com
www.nbcnewyork.com
Conquering the obesity epidemic is the key to our nation's health crisis (which includes corona).Hopeful that perhaps governments and government entities take a fresh look at what the diet and food recommendations have done to obesity this country. Still wondering why a hospital serves patients toast, cereal and orange juice for breakfast.

Doctors Studying Why Obesity May Be Tied to Serious COVID-19
Obesity seems to put people at higher risk for getting very sick if they’re infected with the coronavirus, and doctors are trying to figure out whywww.nbcnewyork.com
LOL Howoritz. He just posted an articles yesterday that 0 college students had been hospitalized due to COVID meanwhile 1 died from it yesterday. SO...... NO MORE HOROWITZ in this thread.
Why do people struggle so much with the Karen meme? Not that difficult.
He just repeats the same lies all over again we debunk them. He goes on Berenson's twitter feed the following day and repeats them all again.With similar restrictions and mask requirements, dipshit. We went over that already and thoroughly debunked your fake talking point.
Looks like the Pfizer vaccine performed well in animal challenge trials. EU also trying to secure 300mil doses.

 www.fiercebiotech.com
www.fiercebiotech.com

Pfizer, BioNTech report 'strong' immune response in animals to COVID-19 mRNA vaccine candidate
Pfizer and its partner BioNTech have five potential COVID-19 vaccines in clinical trials, each of which is based on a different mRNA format. | Pfizer and its partner BioNTech said Wednesday that their most advanced COVID-19 vaccine candidate produced neutralizing antibodies against the virus in...
Derek Lowe weighed in today on the AZ/Oxford vaccine trial halt due the adverse event. The safety board confirmed that the halt is due to a patient receiving the vaccine having a serious adverse event with transverse myelitis, which is the kind of response that has been seen before after vaccinations in rare cases, but the causality has not been yet proven. However, it is an inflammation response, which is not unexpected from introducing a virus into the body and this vaccine uses a modified chimp adeonvirus. There was a second case earlier, but this was deemed to be related to a patient with MS. He thinks the halt was justified and that we need to figure this out - and that if it's one case, making a conclusion will be difficult, but if it's somehow a 2nd case (the MS one), then that could be a major safety issue (excerpt below and a tweet below that from a well known biotech blogger). Nobody said this was going to be easy.Below is what the Times said...sounds like the system is working as it's supposed to and the investigation needs to look into whether the serious adverse event (reportedly spinal inflammation) was vaccine related or not. Most vaccines have rare serious side effects (usually less than 1 in 1 million people), so if it's vaccine related, it could be an issue, but most of the time it's simple coincidence, as it's hard not to have a few out of 30K people in a trial not getting sick. In longer trials for chronic conditions (not vaccines usually, i.e., for things like diabetes or high blood pressure), there are often patients who die and those have to be closely compared for the treatment and the placebo groups.
https://www.nytimes.com/2020/09/08/health/coronavirus-astrazeneca-vaccine-safety.html
A person familiar with the situation, who spoke on the condition of anonymity, said that the participant who experienced the suspected adverse reaction had been enrolled in a Phase 2/3 trial based in the United Kingdom. The individual also said that a volunteer in the U.K. trial had received a diagnosis of transverse myelitis, an inflammatory syndrome that affects the spinal cord and is often sparked by viral infections. However, the timing of this diagnosis, and whether it was directly linked to AstraZeneca’s vaccine, is still unknown.
Transverse myelitis can result from a number of causes that set off the body’s inflammatory responses, including viral infections, said Dr. Gabriella Garcia, a neurologist at Yale New Haven Hospital. But, she added, the condition is often treatable with steroids.
AstraZeneca declined to comment on the location of the participant and did not confirm the diagnosis of transverse myelitis. “The event is being investigated by an independent committee, and it is too early to conclude the specific diagnosis,” the company said. Some said the company’s halt was evidence that the process was working as it should.
“At this stage, we don’t know if the events that triggered the hold are related to vaccination,” said Dr. Luciana Borio, who oversaw public health preparedness for the National Security Council under Mr. Trump and who was acting chief scientist at the F.D.A. under President Barack Obama. “But it is important for them to be thoroughly investigated.”
As an aside, most clinical trials of non-vaccines fail due to efficacy (57%) with only 17% failing due to safety (22% for commercial reasons). I haven't found similar data for vaccines, but I know from my work in the field that vaccines fail at an even lower rate for safety issues, although almost all vaccines have minor to moderate side effects (injection pain, mild fever, headaches, etc.), since they're affecting one's immune system on purpose and the body responds to the "invader" as it should.
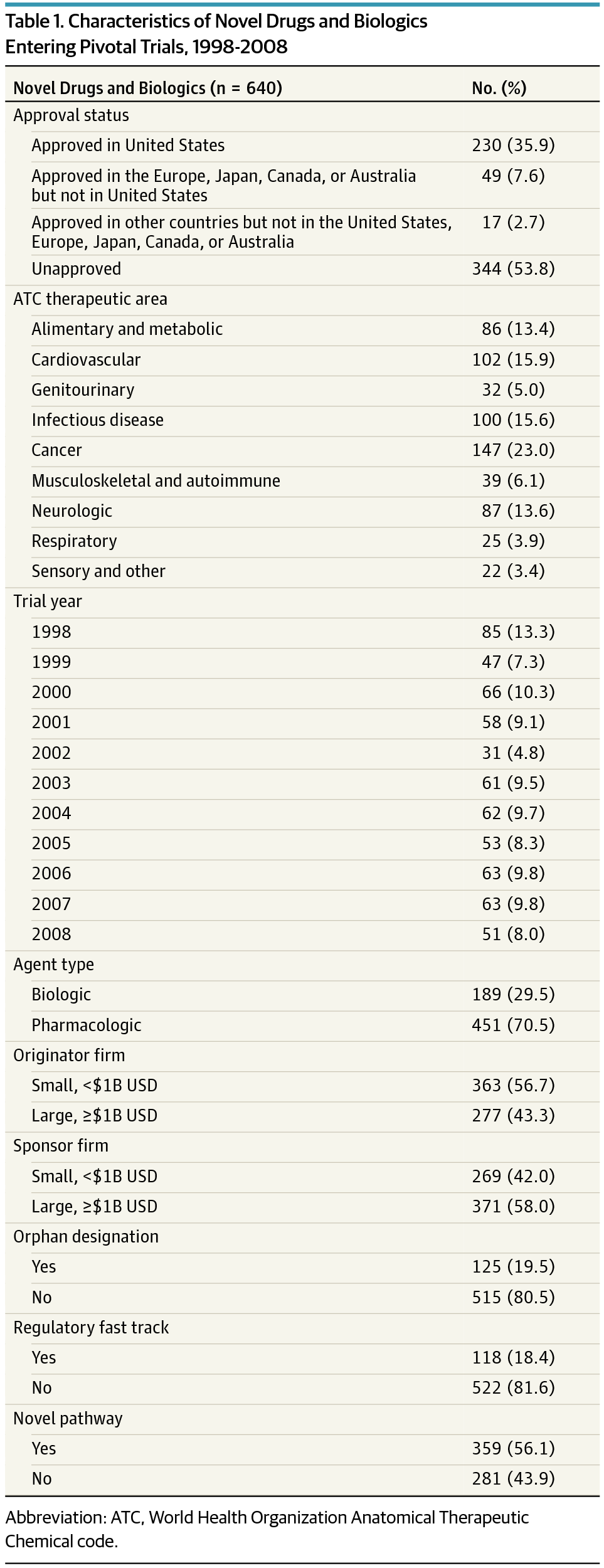
Publication of Drug Failures in Late-Stage Clinical Development
This study assesses factors associated with regulatory approval or reasons for failure of investigational therapeutics in phase 3 or pivotal trials and rates of publication of trial results.jamanetwork.com
AAAS
Unfortunately, it might end up being very difficult to come to a conclusion, especially if this is a single case. (Edit: if this is a second case, though, that will be very bad news indeed) Welcome to vaccine safety trials and the difficulty of working with such data! As mentioned before, since vaccines are designed to be given exclusively to people who are not sick (a very unusual situation in drug development work!), the safety standards have to be very high. But the adverse events themselves (especially the serious ones) can be extremely rare, and the only way to get a statistical foothold on them is to have a very large controlled patient population under study. The Oxford/AstraZeneca trial is enrolling nearly 30,000 people, and the problem is that that may still not be enough for a definite answer on something like this. We’ll all await more information and I’ll revisit this as it becomes available.
He just repeats the same lies all over again we debunk them. He goes on Berenson's twitter feed the following day and repeats them all again.
It's pathetic. Just hoping someone didn't read his last post and doesn't fact check. Then cries about fake news all day. Dishonest hypocrite.
And....its called living. They are living in Switzerland too.
You wondered why one event is being associated with mass spreading and the other isn’t, I gave you an answer. I don’t know what Switzerland or lifestyle choices have to do with it.
lol...that doesnt belong here..omg omfg omg omfg did you see hear what he said, omg omfg
The more interesting headlines coming out of the book are about what people said about him...not things he said.
actually there is nothing interesting coming out of the thousandth book that has come out about Trump, that it moves your needle says something about you
Doubt it moves his or any “libs” needles on Trump. The fact that it and the “thousand” books that came before it haven’t moved your needle is what’s telling.actually there is nothing interesting coming out of the thousandth book that has come out about Trump, that it moves your needle says something about you
You need to expand your horizon - read some Seth Ambramson. Or just read Trump’s non-existent policies on his own website. A man with no plan and an excuse with little hands.
not pewhat does this have to do with the study. Its complete garbage and politically motivated, that its being spread throughout twitter as if true is more proof that the coronavirus is completely political at this point.
They used to watch TRIX cereal commercials as kids. "All part of a balanced breakfast." You are right.Hopeful that perhaps governments and government entities take a fresh look at what the diet and food recommendations have done to obesity this country. Still wondering why a hospital serves patients toast, cereal and orange juice for breakfast.

Doctors Studying Why Obesity May Be Tied to Serious COVID-19
Obesity seems to put people at higher risk for getting very sick if they’re infected with the coronavirus, and doctors are trying to figure out whywww.nbcnewyork.com
I think a major factor is that healthier foods cost more to purchase, prepare, and store.
Well the Russians are trying their best to win the PR race, but they've failed, miserably, from a scientific and public health safety perspective. They're "approving" a vaccine that hasn't even started phase III, large scale clinical trials yet - you know, the ones required to determine safety and efficacy. It's possible their approach, a mixture of two human adenovirus vectors (the CanSino approach, although they use just one adenovirus; also the Oxford/Astra-Zeneca approach uses a single chimp adenovirus vector) can work, but the issue is having done so little work so far and saying it's "approved," although they don't intend a general rollout until October, when it's possible they'll have a read from phase III trials. No way in hell I'd be taking that vaccine with so little data. As is often the case, Derek Lowe chimed in with a timely blog entry on the Russian vaccine today.
https://www.reuters.com/article/us-...tent&utm_medium=trueAnthem&utm_source=twitter
https://blogs.sciencemag.org/pipeline/archives/2020/08/11/the-russian-vaccine
Many will have heard Russia’s announcement that they have approved a coronavirus vaccine. I’ve already had several people ask me what I think of it, so let me be clear: I think it’s a ridiculous publicity stunt. If it’s supposed to make Russia look like some sort of biotechnology powerhouse, then as far as I’m concerned it does the opposite. It makes them look desperate, like the nation-state equivalent of a bunch of penny-stock promoters. The new airliner design prototype just got off the ground – time to sell tickets and load it full of passengers, right?
Why so negative? Look at what’s being claimed – the first coronavirus vaccine to receive regulatory approval. But “regulatory approval” is not some international gold standard, and these sorts of decisions show you why. Let’s be honest: there is no way that you can responsibly “approve” a vaccine after it’s only been into human trials for what numerous reports say is less than two months. That’s about enough time to do the first steps, a Phase I trial that gives you some idea of immune response across more than one dose. It is simply not enough time to do a reasonable efficacy workup as well, and absolutely not enough time to get any sort of reading on safety. Here’s a good article going into those timelines in more depth.
Some clear irregularities in the paper published recently in the Lancet by the Russian vaccine research team, on the vaccine that the Russian government said was "approved" before phase III trials were even started, which have been called out as a "note of concern" by many prominent experts in the field.
Basically, the authors of the note call out the observation that there are repeated data patterns for individual patients in multiple figures in the paper. This is true for both discrete variables (where it's more possible, but still unlikely) and for continuous variables (like the CD4 cellular response to the vaccine), where exact repeats should almost never occur.
Doesn't inspire confidence in the integrity of their efforts or vaccine. Will be interesting to see the response from the authors and the Lancet, which recently got a black eye from the Surgisphere data souce mess on the HCQ and ACE papers a few months ago.

Note of concern
Open letter to DY Logunov et al., authors of: “Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised ph…
 cattiviscienziati.com
cattiviscienziati.com
actually there is nothing interesting coming out of the thousandth book that has come out about Trump, that it moves your needle says something about you
I forgot it’s filled with lies from radical leftists like Dan Coats, General Mattis, and uhh Trump himself.
No joke I work with a guy who called Trump a Clinton plant meant to sabotage the GOP until Trump actually won, then became part of the “any critique of Trump is a deep-State takedown” crowd. Funny how that happens.
Anyways, put on your mask and go outside, winter is coming.
I forgot it’s filled with lies from radical leftists like Dan Coats, General Mattis, and uhh Trump himself.
No joke I work with a guy who called Trump a Clinton plant meant to sabotage the GOP until Trump actually won, then became part of the “any critique of Trump is a deep-State takedown” crowd. Funny how that happens.
Anyways, put on your mask and go outside, winter is coming.
#unmask when outside
Does each of Q’s books contain thousands Of citations fact-checked by teams of professional fact-checkers?omg Seth Abrahamson...thats Q level stuff, i am not joking

Vitamin D deficiency raises COVID-19 infection risk by 77%, study finds - UPI.com
Vitamin D deficiency increases a person's risk for catching COVID-19 by 77% compared to those with sufficient levels of the nutrient, a study published Thursday by JAMA Network Open found.
 www.upi.com
www.upi.com
Vitamin D deficiency raised infection risk by 77%
A lot of people have Vitamin D deficiencies. Pretty common and most people don’t even know it unless they get detailed annual bloodwork.
#unmask when outside
True, I don’t wear a mask outside unless intending to interact with others
A lot of people have Vitamin D deficiencies. Pretty common and most people don’t even know it unless they get detailed annual bloodwork.
Serious question, do people that consume a lot of adult beverages have low Vitamin D levels?
Serious question, do people that consume a lot of adult beverages have low Vitamin D levels?
Pretty sure that doesn’t help. Think my Dr told me that after I told him the truth to the “how often do you drink” question when I was in college 😂
Pretty sure that doesn’t help. Think my Dr told me that after I told him the truth to the “how often do you drink” question when I was in college 😂
Thanks, I'll tell my cousin to slow it down a little
Hmmmm, I actually thought he was a Delusional Uninformed Moronic Bro Conservative Unintelligent Nonsensical Troll.
Another high and mighty fraud on display
Does each of Q’s books contain thousands Of citations fact-checked by teams of professional fact-checkers?
I dont know but i do know Seth got trapped in a Russian rabbit hole of wrong as did many here
Yes most adults over 55 are vitaminD deficient to some extent. During the winter months especially in the colder climates of the north , northeast getting sunlight and eating a diet high in vitamin D is lacking . Makes sense when you read up on it and see what happened in the NJ/NY metro northeast. Nursing homes and Assisted Living Facilities would lend to those beliefs that this lack of sunlight and poor vitamin intake exacerbates the chance for more severe cases. The add the obesity issue and it’s a perfect storm.A lot of people have Vitamin D deficiencies. Pretty common and most people don’t even know it unless they get detailed annual bloodwork.
Just like I wouldn't take a single poll without a grain of salt, I question Bolton's motivations. However, when taking all of the books and articles that confirm much of the same sentiment about Trump, the picture as a composite is likely accurate.Has anyone read Bolton’s book? I have, it’s unbelievable and frightening. I haven’t heard a peep from any Trump supporters. About the same amount as Trump’s criticisms of Russia/Putin. Full disclosure, I voted for Trump in 2016. Not leaning that way this time around.
True that. I had it back in 2014. Getting pains and weakness in my calf muscles. Dr. said take D. Started taking 5K IU every night. Pain and weakness gone in about three to four weeks. Still taking same dose. Thanks for posting.A lot of people have Vitamin D deficiencies. Pretty common and most people don’t even know it unless they get detailed annual bloodwork.
Just for clarification, Bolton is not voting for Biden or Trump. He’s writing in a candidate. I only brought his book up because no one is to the right of Bolton and he was appalled at how dysfunctional the WH isJust like I wouldn't take a single poll without a grain of salt, I question Bolton's motivations. However, when taking all of the books and articles that confirm much of the same sentiment about Trump, the picture as a composite is likely accurate.
Reported.Hmmmm, I actually thought he was a Delusional Uninformed Moronic Bro Conservative Unintelligent Nonsensical Troll.
Unprovoked and disgusting personal attack on another poster.
Reported.
Unprovoked and disgusting personal attack on another poster.
You really shouldn't advertise what a spineless, little rat you are. Only reflects poorly on you.
John Bolton is a scumbag, why would I care a lick about his book.Has anyone read Bolton’s book? I have, it’s unbelievable and frightening. I haven’t heard a peep from any Trump supporters. About the same amount as Trump’s criticisms of Russia/Putin. Full disclosure, I voted for Trump in 2016. Not leaning that way this time around.
- Status
- Not open for further replies.
Similar threads
- Replies
- 44
- Views
- 2K
- Replies
- 472
- Views
- 18K
- Replies
- 25
- Views
- 2K
- Replies
- 254
- Views
- 12K
- Replies
- 17
- Views
- 1K
ADVERTISEMENT
Latest posts
-
Basketball Rutgers Basketball to return to Players Era Festival in 2025
- Latest: RUSCFORMERLYRULOU
-
-
-
ADVERTISEMENT